DUBLIN, Nov. 22, 2016 /PRNewswire/ -- Adapt Pharma welcomes the U.S. Food and Drug Administration's (FDA) Consumer Update What to Ask Your Doctor Before Taking Opioids. Adapt applauds the FDA for its specific recommendation that opioid consumers ask their provider "Can I have a Prescription for Naloxone?"
The Consumer Update suggests that before taking opioids consumers " should discuss with your doctor whether you should also receive a prescription for naloxone, a drug that can reverse the effects of an opioid overdose and could save lives. In many cases it makes sense to be prepared for potential problems by keeping naloxone in your home."
Adapt encourages consumers to talk to their healthcare providers about naloxone, particularly if they or a loved one is prescribed opioids. Adapt Pharma hopes this update will continue to promote conversations of the availability of FDA-approved naloxone products, like NARCAN® Nasal Spray, which are designed specifically with the community in mind and require no medical experience or formal training to use.
"The FDA has already taken tremendous steps in response to the country's opioid overdose epidemic, specifically through its expedited approval and support of access to community-ready naloxone products like NARCAN® Nasal Spray," said Seamus Mulligan, CEO of Adapt Pharma. "Adapt Pharma welcomes this most recent example of the FDAs efforts to combat the epidemic."
"We've worked closely with state and local governments to expand access to NARCAN® Nasal Spray across the country," said Mike Kelly, President of U.S. Operations at Adapt Pharma. "Additionally, in many states you can walk into many local or chain pharmacies and pick up our product without a physician's prescription and Narcan is covered by most insurance plans."
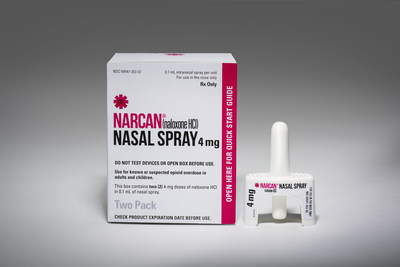
NARCAN® Nasal Spray is the first and only FDA-approved naloxone nasal spray for the emergency treatment of opioid-, fentanyl- and heroin-related overdose. It is now available as a ready-to-use, needle-free, 4 mg concentrated dose of naloxone in a single spray. As the first and only FDA-approved naloxone nasal spray, NARCAN® Nasal Spray provides a ready-to-use alternative to currently available opioid overdose emergency treatments. NARCAN® Nasal Spray is not a substitute for emergency medical care. Please see Indications and Important Safety Information below.
ABOUT NARCAN® (naloxone HCl) NASAL SPRAY
NARCAN® Nasal Spray is indicated for the emergency treatment of known or suspected opioid overdose, as manifested by respiratory and/or central nervous system depression.
NARCAN® Nasal Spray is intended for immediate administration as emergency therapy in settings where opioids may be present.
NARCAN® Nasal Spray is not a substitute for emergency medical care. Always seek emergency medical assistance in the event of a suspected, potentially life-threatening opioid emergency after administration of the first dose of NARCAN® nasal spray.
If the desired response is not obtained after 2 or 3 minutes, administer an additional dose of NARCAN® Nasal Spray using a new NARCAN® Nasal Spray. If the patient responds to NARCAN® Nasal Spray and relapses back into respiratory depression before emergency assistance arrives, administer an additional dose and continue surveillance of the patient. If there is still no response and additional doses are available, administer additional doses of NARCAN® Nasal Spray every 2 to 3 minutes using a new NARCAN® Nasal Spray with each dose until emergency medical assistance arrives. Additional supportive and/or resuscitative measures may be helpful while awaiting emergency medical assistance.
Please see Indications and Important Safety Information below.
Please see full prescribing information for NARCAN® Nasal Spray, available at http://www.NARCANnasalspray.com/pdf/NARCAN-Prescribing-Information.pdf
AVAILABILITY OF NARCAN® NASAL SPRAY
NARCAN® Nasal Spray was made available in February of 2016.
Qualifying group purchasers may source NARCAN® Nasal Spray directly from wholesalers and distributors. To place a pre-order immediately or for assistance in sourcing NARCAN® Nasal Spray please contact Adapt Pharma's dedicated Customer Service Team at 844-4-NARCAN® (844-462-7226) or email customerservice@adaptpharma.com.
NARCAN® NASAL SPRAY INDICATIONS AND IMPORTANT SAFETY INFORMATION
Indications
NARCAN® (naloxone hydrochloride) Nasal Spray is an opioid antagonist indicated for the emergency treatment of known or suspected opioid overdose, as manifested by respiratory and/or central nervous system depression. NARCAN® Nasal Spray is intended for immediate administration as emergency therapy in settings where opioids may be present.
NARCAN® Nasal Spray is not a substitute for emergency medical care.
Important Safety Information
NARCAN® Nasal Spray is contraindicated in patients known to be hypersensitive to naloxone hydrochloride.
Seek emergency medical assistance immediately after initial use, keeping the patient under continued surveillance.
Risk of Recurrent Respiratory and CNS Depression: Due to the duration of action of naloxone relative to the opioid, keep the patient under continued surveillance and administer repeat doses of naloxone using a new nasal spray with each dose, as necessary, while awaiting emergency medical assistance.
Risk of Limited Efficacy with Partial Agonists or Mixed Agonists/Antagonists: Reversal of respiratory depression caused by partial agonists or mixed agonists/antagonists, such as buprenorphine and pentazocine, may be incomplete. Larger or repeat doses may be required.
Precipitation of Severe Opioid Withdrawal: Use in patients who are opioid dependent may precipitate opioid withdrawal characterized by body aches, diarrhea, increased heart rate (tachycardia), fever, runny nose, sneezing, goose bumps (piloerection), sweating, yawning, nausea or vomiting, nervousness, restlessness or irritability, shivering or trembling, abdominal cramps, weakness, and increased blood pressure. In neonates, opioid withdrawal may be life-threatening if not recognized and properly treated and may be characterized by convulsions, excessive crying, and hyperactive reflexes. Monitor for the development of opioid withdrawal.
Risk of Cardiovascular (CV) Effects: Abrupt postoperative reversal of opioid depression may result in adverse CV effects. These events have primarily occurred in patients who had pre-existing CV disorders or received other drugs that may have similar adverse CV effects. Monitor these patients closely in an appropriate healthcare setting after use of naloxone hydrochloride.
The following adverse reactions were observed in a NARCAN Nasal Spray clinical study: increased blood pressure, musculoskeletal pain, headache, nasal dryness, nasal edema, nasal congestion, and nasal inflammation.
See Instructions for Use and full prescribing information in the use of this product, available here: http://www.narcannasalspray.com/pdf/NARCAN-Prescribing-Information.pdf.
Additional information, including full prescribing information for NARCAN® Nasal Spray, and important safety information and instructions for use, is also available at www.NARCAN®NasalSpray.com.
To report SUSPECTED ADVERSE REACTIONS, contact Adapt Pharma, Inc. at 1-844-4NARCAN® (1-844-462-7226) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
ABOUT ADAPT PHARMA
Adapt Pharma is a privately-held pharmaceutical company committed to positively impacting the lives of patients. Adapt Pharma's strategy is to identify, evaluate, selectively acquire and enhance the value of late stage development, and FDA approved, pharmaceutical products. Adapt Pharma's company headquarters is in Dublin, Ireland and its U.S. headquarters is in Radnor, Pennsylvania. For more information, please visit www.adaptpharma.com.
MEDIA CONTACT INFORMATION
Thom Duddy
Executive Director, Communications
Adapt Pharma
thomas.duddy@adaptpharma.com
484-532-5470

Photo - http://photos.prnewswire.com/prnh/20160712/388735
Logo - http://photos.prnewswire.com/prnh/20160410/353441LOGO
To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/adapt-pharma-welcomes-us-food-and-drug-administration-consumer-update-regarding-opioids-300367595.html
SOURCE Adapt Pharma





