Abiomed, part of Johnson & Johnson MedTech1, announced that novel data from seven research studies focused on optimizing outcomes for patients receiving Impella-supported high-risk percutaneous coronary intervention (HRPCI) will be presented at the 35th Transcatheter Cardiovascular Therapeutics (TCT) annual scientific symposium taking place in San Francisco between Oct. 24 - 26.
|
|
| [23-October-2023] |
|
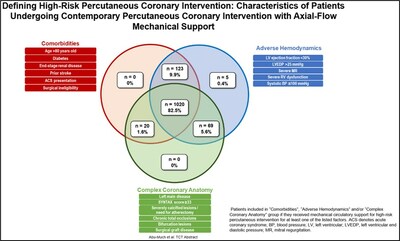
DANVERS, Mass., Oct. 23, 2023 /PRNewswire/ -- Abiomed, part of Johnson & Johnson MedTech1, announced that novel data from seven research studies focused on optimizing outcomes for patients receiving Impella-supported high-risk percutaneous coronary intervention (HRPCI) will be presented at the 35th Transcatheter Cardiovascular Therapeutics (TCT) annual scientific symposium taking place in San Francisco between Oct. 24 - 26. As part of an academic collaboration coordinated by the Cardiovascular Research Foundation (CRF)22, researchers conducted new analyses of data from the prospective, multicenter PROTECT III study, which includes the most robust contemporary data set of 1,237 Impella-supported HRPCI patients treated at 46 sites in the U.S. between March 2017- March 2020. IMPELLA DATA PRESENTATIONS The seven studies include an analysis of the characteristics of PROTECT III patients that demonstrates how physicians are distinguishing which patients are high-risk and appropriate for Impella-supported HRPCI procedures. The data shows that 82.5 percent of patients have features in all three high-risk domains: comorbidities, complex coronary anatomy and adverse hemodynamics. It also demonstrates that 99.6 percent of patients have risk factors from two or more of the high-risk domains. (see figure 1) Arsalan Abu-Much, MD, a cardiologist and postdoctoral fellow at CRF, will present this abstract (#TCT 216) on Thursday, Oct. 26 at 11:18 a.m. PDT at Station 6 – Emerging Clinical Science & Research, Hall C, Exhibition Level, Moscone South. Additional Impella HRPCI abstracts being presented on Oct. 26 include (in order of presentation timing):
DAILY RECAP WEBCAST
Abiomed at TCT 2023
About Abiomed About Johnson & Johnson MedTech Cautions Concerning Forward-Looking Statements This press release contains "forward-looking statements" as defined in the Private Securities Litigation Reform Act of 1995 regarding Abiomed technology. The reader is cautioned not to rely on these forward-looking statements. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or known or unknown risks or uncertainties materialize, actual results could vary materially from the expectations and projections of Abiomed, Inc. and/or Johnson & Johnson. Risks and uncertainties include, but are not limited to: uncertainty of commercial success; challenges to patents; competition, including technological advances, new products and patents attained by competitors; manufacturing difficulties and delays; product efficacy or safety concerns resulting in product recalls or regulatory action; changes to applicable laws and regulations, including global health care reforms; changes in behavior and spending patterns of purchasers of health care products and services; and trends toward health care cost containment. A further list and descriptions of these risks, uncertainties and other factors can be found in Johnson & Johnson's Annual Report on Form 10-K for the fiscal year ended January 1, 2023, including in the sections captioned "Cautionary Note Regarding Forward-Looking Statements" and "Item 1A. Risk Factors," and in Johnson & Johnson's subsequent Quarterly Reports on Form 10-Q and other filings with the Securities and Exchange Commission. Copies of these filings are available online at www.sec.gov, www.jnj.com or on request from Johnson & Johnson. None of Abiomed, Inc. nor Johnson & Johnson undertakes to update any forward-looking statement as a result of new information or future events or developments. For further information please contact: Jenny Leary 1 Abiomed, Inc. is part of Johnson & Johnson MedTech, which comprises the surgery, orthopedics, vision and interventional solutions businesses within Johnson & Johnson's MedTech segment.
SOURCE Abiomed |