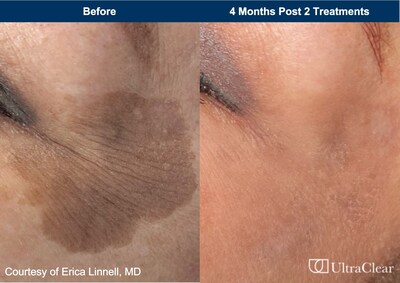
Acclaro Medical, a medical technology company that specializes in developing and delivering trailblazing solutions for medical aesthetics and surgical practitioners worldwide, today announced clearance by the U.S. Food and Drug Administration (FDA) for a new indication of the Company’s UltraClear® cold ablative fractional 2910 nm fiber laser.
| ~ The Global Market for Pigmented Lesion Treatment Is Expected to Reach $3.37 Billion in 2024, and $4.69 Billion by 20291 ~ Leading Experts Will Illuminate Clinical Efficacy of UltraClear’s New Indications at the Upcoming SCALE Symposium for Aesthetic Medicine and Laser Education ~ SMITHFIELD, R.I., May 14, 2024 /PRNewswire/ -- Acclaro Medical, a medical technology company that specializes in developing and delivering trailblazing solutions for medical aesthetics and surgical practitioners worldwide, today announced clearance by the U.S. Food and Drug Administration (FDA) for a new indication of the Company’s UltraClear® cold ablative fractional 2910 nm fiber laser. The 510K approval expands the use of UltraClear’s novel technology for the treatment of benign pigmented lesions and vascular dyschromia. “This clearance underpins our commitment to addressing the unmet needs of patients living with pigmentation disorders by improving upon the current treatment paradigm of acids, peels and passé devices,” said Shlomo Assa, Co-Founder and President, Chief Technology Officer of Acclaro Medical. “Whether troubled by benign brown lesions or red discoloration, patients can now experience UltraClear’s proven ability to rejuvenate their physical appearance with excellent results, reduced discomfort, minimal downtime and very limited risk.” “Any change in skin tone can be concerning or upsetting,” noted Dr. Erica Linnell, Founder MD of Linnell Dermatology and Aesthetics, Seattle, WA. “With the heightened awareness of skin cancer checks and an aging population, we have seen a corresponding rise in patient visits for evaluation and treatment of their pigmented lesions. The expanded regulatory indication for the UltraClear fiber laser now paves the way for us to resurface and make a difference in improving pigmentation issues with just one to two treatments.” At the upcoming SCALE meeting in Nashville, TN, on May 16-19, 2024, key opinion leaders will showcase the effectiveness and versatility of UltraClear to treat a vast array of aesthetic skin conditions, ranging from the early signs of aging, facial wrinkles and crinkles around the eyes to unwanted pigmentation, the effects of gravity and scars, including acne, surgical and traumatic scars. By stimulating new cell growth leading to collagen and elastin remodeling, UltraClear treatment produces smoother and healthier-looking skin with minimal medical downtime. Its advanced pulsing and fractionated technology, which perfectly balances cold and thermal energy, is suitable for all skin types and may not require topical numbing, providing a rewarding patient experience for numerous aesthetic concerns on patient of all skin colors. Visit Exhibit Tabletop #808 to learn more about UltraClear at SCALE. PODIUM PRESENTATIONS AT SCALE: Friday, May 17th Friday, May 17th Saturday, May 18th About Acclaro Medical 1 Pigmented Lesion Treatment Market Size & Share Analysis - Growth Trends & Forecasts (2024 - 2029) For media inquiries, contact:
SOURCE Acclaro Medical Corporation |