AOBiome Therapeutics, Inc., a leading clinical-stage microbiome company focusing on inflammatory conditions, recently completed a Phase 2b study of 547 patients with moderate-to-severe itch and mild-to-moderate atopic dermatitis.
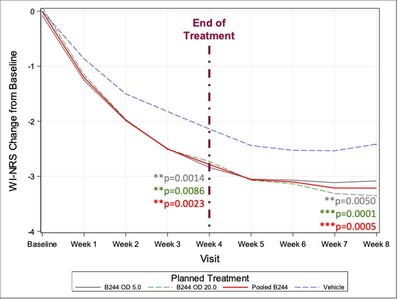
- The additional study showed the MoA related to patients’ continued improvement of itch over time as also observed in the Phase 2b study, which saw an increase from 34.0% at 4 weeks to 40.9% itch reduction at 8 weeks.
- The study further demonstrates the additional longer-term treatment benefits of the AOB’s cellular structure on itch, even when the bacteria is metabolically inactive (without ammonia processing or production of nitric oxide and nitrite).
- Additionally, reductions in itch were seen within the first hour of application.
CAMBRIDGE, Mass., Sept. 20, 2022 /PRNewswire/ -- AOBiome Therapeutics, Inc. (“AOBiome”), a leading clinical-stage microbiome company focusing on inflammatory conditions, recently completed a Phase 2b study of 547 patients with moderate-to-severe itch and mild-to-moderate atopic dermatitis. The trial results showed continued durability of treatment effect and separation from placebo post-trial of 40.9% reduction in itch from baseline at 8 weeks, 4 weeks after the last dose.
Further investigation supported previous findings on the secondary effects of inert Ammonia Oxidizing Bacteria (AOB), even after it no longer converts ammonia to Nitric Oxide and Nitrite. A recently-published in vitro study has demonstrated that both live and metabolically inactive AOB can downregulate Th2-associated pruritic cytokines. For more details see: https://www.nature.com/articles/s41598-021-93299-1.
The company ran a separate open label 30 subject cosmetic safety study utilizing a cosmetic strength of metabolically inactive (heat-killed) AOB in subjects with itch associated with mild-to-moderate atopic dermatitis. The results of this study bolster the assessment of AOB as it relates to both safety and the positive results seen in the company’s 547 patient Phase 2b study with live B244 material and the trailing effect, once that study was completed.
Key results from the separate Cosmetic study:
- No adverse events
- Reductions in itch were seen within the first hour of application
- Itch score improvements were observed in both adult and pediatric subjects throughout the study by VAS (0-10 scale) and Itch Man (0-4 scale), respectively. Mean adult itch scores improved from 6.98±1.78 at Baseline to 3.01±2.22 at Day 14 (57% reduction), and mean pediatric itch scores improved from 2.64±0.67 at Baseline to 0.82±0.75 at Day 14 (69% reduction)
- 57.9% of adult subjects showed a 4-point improvement in itch (Visual Analog Scale) at Day 14 (11-point scale)
- 81.8% of pediatric subjects showed a 2-point improvement in itch (Itch Man Scale) at Day 14 (5-point scale)
- The benefits for itch were sustained, with reductions from Baseline persisting after discontinuation of use – similar to results seen in the Phase 2b study.
- Subjects showed improvement in the appearance of eczema at Day 14 as compared to Baseline.
“These results show that AOB can still have real-world impact on the immune system even when AOB is neither consuming ammonia or no longer viable. This has huge implications for AOB’s persistence on the skin, the effectiveness of our clinical products and the simplicity of incorporating AOB into patient’s everyday routines.” says President & CEO, Todd Krueger.
AOBiome is in the process of planning Phase 3 studies for B244, for which it aims to start recruiting patients in 2023.
AOBiome’s B244 platform is a patented, proprietary, topical formulation. Once deployed, B244 produces nitric oxide, a signaling molecule known to regulate inflammation and vasodilation. B244 has been observed to be well-tolerated in clinical studies to date.
Additionally, recently published immunology data demonstrates that B244 can reduce the inflammatory and pruritic cytokines IL-4, IL-5, IL-13, and IL-31. See full article at: https://www.nature.com/articles/s41598-021-93299-1.
AOBiome Therapeutics, Inc. is a Cambridge, MA-based life sciences company focused on transforming human health by developing topical therapeutics for inflammatory conditions. AOBiome is advancing a pipeline of multiple, clinical-stage therapeutic candidates. Learn more at www.aobiome.com.
Contacts:
For Media Inquiries:
Jim Hoffman
845-417-3487
Press@AOBiome.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/additional-study-augments-the-results-of-aobiomes-successful-547-patient-phase-2b-trial-for-the-treatment-of-mild-to-moderate-atopic-dermatitis-and-moderate-to-severe-pruritus-using-its-b244-investigational-drug-301627623.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/additional-study-augments-the-results-of-aobiomes-successful-547-patient-phase-2b-trial-for-the-treatment-of-mild-to-moderate-atopic-dermatitis-and-moderate-to-severe-pruritus-using-its-b244-investigational-drug-301627623.html
SOURCE AOBiome