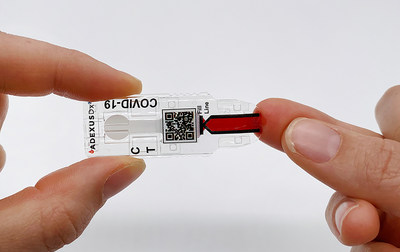
NOWDiagnostics, Inc. announced today that it has received Emergency Use Authorization (EUA) for its ADEXUSDx® COVID-19 antibody test for use in moderate-complex settings and at the point-of-care from the U.S. Food and Drug Administration (FDA)
|
SPRINGDALE, Ariz., May 26, 2021 /PRNewswire/ -- NOWDiagnostics, Inc. announced today that it has received Emergency Use Authorization (EUA) for its ADEXUSDx® COVID-19 antibody test for use in moderate-complex settings and at the point-of-care from the U.S. Food and Drug Administration (FDA). Following the EUA, NOWDiagnostics will begin offering the ADEXUSDx® COVID-19 Test for use across a variety of CLIA-waived health care settings including pharmacies, clinics, and hospital emergency rooms, while studies are ongoing to make this revolutionary and patented technology available for over-the-counter detection of SARS-CoV-2 antibodies. The rapid-results fingerstick test is the only emergency use authorized, self-contained assay available that measures the presence of COVID-19 antibodies to deliver accurate, reliable, and affordable results in 15 minutes. Performance of the test does not require a phlebotomist, buffers, reagents, or additional equipment. The application to use the ADEXUSDx® COVID-19 Test for moderate complexity testing was originally submitted to the FDA in May 2020. In August 2020, the Biomedical Advanced Research and Development Authority (BARDA), part of the Office of the Assistant Secretary for Preparedness and Response at the U.S. Department of Health and Human Services, provided funding and technical support to NOWDiagnostics for the development of the ADEXUSDx® COVID-19 Test. Point of care (CLIA Waiver) performance data were subsequently submitted to the FDA in November 2020 to expand the use of the test. Kevin Clark, Chief Executive Officer of NOWDiagnostics, Inc., said, "The ADEXUSDx® COVID-19 Test is literally a lab at the tip of your finger, specifically designed to make diagnostic testing possible at home. This EUA approval is the first step in making that a reality. The ADEXUSDx® COVID-19 Test uses the same platform as our other FDA-cleared and/or CE marked, next-generation tests which are affordable, portable, and deliver laboratory-quality results in minutes without any additional supplies. We believe antibody testing is essential to America's recovery from the COVID-19 pandemic, especially with the ongoing roll out of vaccines, and we're proud to be responding to the urgent need for a reliable COVID-19 antibody test that targets the spike protein with our made-in-the-U.S.A. product." Developed and manufactured at NOWDiagnostics' Springdale, Arkansas facility with materials sourced from American suppliers, the ADEXUSDx® COVID-19 Test uses a drop (40 μL) of fingerstick or venous whole blood, serum, or plasma to identify individuals with SARS-CoV-2 antibodies. Employing sophisticated separation microfluidics and proprietary multi-layer membranes, the ADEXUSDx® COVID-19 Test separates plasma from whole blood automatically and, in minutes, displays results indicating whether the subject has developed SARS-CoV-2 antibodies for COVID-19. Additionally, the ADEXUSDx® COVID-19 antibody test targets antibodies to the spike protein of the SARS-CoV-2 virus, positioning it to provide important utility in a post-vaccination world. The features of the self-contained ADEXUSDx® COVID-19 Test, from its simplicity to its portability, make it uniquely suited for large-scale and remote testing—from under resourced areas lacking access to traditional health care laboratories and facilities to more austere environments like those encountered by the military or aid workers. This project has been funded in part with federal funds from the Department of Health and Human Services, Office of the Assistant Secretary for Preparedness and Response; Biomedical Advanced Research and Development Authority, under contract number 75A50120C00156. Laboratories or health care providers may visit www.c19development.com/order to place orders. NOWDiagnostics, Inc., based in Springdale, Arkansas, is a leader in innovative diagnostics testing. Its ADEXUSDx® product line features a lab at the tip of your finger, using a single drop of blood to test for a variety of common conditions, illnesses, and diseases, with results in a matter of minutes. By eliminating the need to send tests to off-site laboratories, NOWDiagnostics products have the potential to decrease the waiting period to determine test results by days. For more information about NOWDiagnostics, visit www.nowdx.com. For more information about the ADEXUSDx® COVID-19 Test, including its intended use, features, benefits, and directions for use, visit www.c19development.com. The ADEXUSDx® COVID-19 Test will be distributed by C19 Development, LLC, a wholly owned subsidiary of NOWDiagnostics. Laboratories may contact www.c19development.com/order to place an order.
SOURCE NOWDiagnostics |