Aerin Medical Inc. today announced the publication of positive two-year follow-up data from the AERWAY trial for patients with severe or extreme nasal airway obstruction (NAO) due to nasal valve collapse (NVC).
Building upon a growing body of evidence, AERWAY demonstrates significant and sustained improvements, including for nasal valve collapse patients with untreated septal deviations and either static or dynamic nasal valve collapse
MOUNTAIN VIEW, Calif.--(BUSINESS WIRE)-- Aerin Medical Inc., a company dedicated to providing Ear, Nose and Throat (ENT) physicians with non-invasive solutions for the treatment of chronic nasal conditions, today announced the publication of positive two-year follow-up data from the AERWAY trial for patients with severe or extreme nasal airway obstruction (NAO) due to nasal valve collapse (NVC). Results featured in Laryngoscope Investigative Otolaryngology showed a significant and long-term improvement in NAO symptom burden.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20230713117673/en/
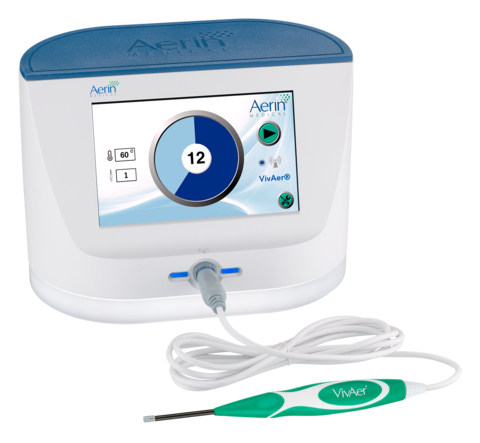
Two-year data published online in Laryngoscope Investigative Otolaryngology showed a statistically significant and sustained improvement in the symptom burden of those with nasal airway obstruction following a single treatment with VivAer®. A non-invasive technology developed by Aerin Medical Inc., VivAer uses patented, temperature-controlled radiofrequency energy to provide long-term relief from nasal obstruction. (Photo: Business Wire)
The AERWAY outcomes demonstrated the ongoing effectiveness and safety of VivAer, with treated patients reporting sustained two-year symptom improvement that remained consistent with three-month, six-month and one-year results. Key findings include:
- Statistically significant and sustained improvement in symptom burden was achieved in all subpopulations including NVC patients with untreated septal deviations and either static or dynamic NVC
- 90.1% of the patients were responders and improvements in total Nasal Obstruction Symptom Evaluation (NOSE) Scale scores were maintained from time of treatment through two years
- Usage of oral medications, nasal sprays and breathing strips decreased at two years compared to baseline
- Treatment with VivAer was well-tolerated, with no serious adverse events related to the procedure and/or device
“The AERWAY long-term data showed that VivAer delivered relief to 90% of patients suffering from NAO,” said William Yao, M.D., AERWAY’s principal investigator from the Department of Otorhinolaryngology - Head and Neck Surgery at McGovern Medical School at UTHealth Houston. “These results demonstrate that VivAer offers a useful option for otolaryngologists and patients, as the evidence showed statistically significant and sustained improvement even for patients with other untreated anatomical factors like septal deviation.”
Earlier this year the American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) issued a position statement noting that NVC is a structural issue that cannot effectively be handled with medical treatment (i.e., intranasal steroids). The Academy notes that office-based techniques like radiofrequency treatment can stabilize the nasal valve, optimize NVC patient outcomes, and be performed either as a standalone procedure or in combination with other procedures to potentially avoid multiple surgeries.
The AERWAY study, a prospective, multi-center trial, followed patients after a single treatment session with VivAer and collected two-year outcomes for 91 patients. Before treatment, all participants exhibited extreme or severe NAO, based on the validated NOSE Scale score, with NVC as a primary contributor to their symptoms.
“The positive two-year outcomes from the AERWAY trial build upon published VivAer clinical results, including superior symptom improvements in a randomized controlled trial and a four-year study showing long-term durability of a single VivAer treatment,” said Matt Brokaw, CEO of Aerin Medical. “We are proud to see the growing body of evidence that demonstrates the safety, effectiveness and predictability of VivAer for patients suffering from NAO due to NVC, and we thank the numerous physician investigators who have confirmed benefits for their patients.”
About Nasal Airway Obstruction (NAO)
The most common symptoms of NAO include nasal congestion or stuffiness, trouble breathing through the nose, trouble sleeping, and difficulty breathing well during exercise or exertion, and these symptoms can take a heavy toll on daily life. NVC contributes to nasal obstruction for 73% of highly symptomatic individuals,1 but is often under-diagnosed and left untreated. This can have a significant impact on a person’s quality of life, as the nasal valve is the narrowest part of the nasal airway and even small obstructions can create an exponential reduction in airflow. VivAer offers ENT physicians a unique, non-invasive and office-friendly option that durably remodels tissue in the nasal valve to address NAO in appropriate patients.
About VivAer
VivAer is a non-invasive technology that uses patented, temperature-controlled radiofrequency energy and is clinically demonstrated to provide long-term relief from nasal obstruction. VivAer features a thin, wand-like stylus that attaches to a console. The stylus is inserted via the nostril to gently remodel the nasal tissue and improve airflow. VivAer does not involve any cutting, removal of nasal tissue or bone, or the use of an implant. Treatment with VivAer may be performed during an office visit with local anesthesia. Patients typically experience little discomfort with minimal to no downtime and are often able to return to normal activities following treatment. The VivAer Stylus received CE Mark in 2016 and FDA 510(k) clearance in December 2017. For more information, visit www.VivAer.com.
About Aerin Medical
Aerin Medical is a privately held, venture-backed company, with U.S. offices in California and Texas. Aerin’s mission is to provide ENT physicians with non-invasive solutions for the treatment of chronic nasal conditions. The company’s products, VivAer® for nasal airway obstruction and RhinAer® for chronic rhinitis, leverage Aerin’s proprietary temperature-controlled technology, which allows ENT physicians to reliably improve patients’ symptoms with in-office procedures performed under local anesthesia. More than 75,000 patients have been treated with Aerin Medical products to date. For more information, please visit www.aerinmedical.com and follow Aerin Medical on Facebook, Twitter, Instagram, LinkedIn and YouTube.
1 Clark, David; Del Signore, Anthony; Raithatha, Roheen; Senior, Brent; 2018. Nasal airway obstruction: Prevalence and anatomic contributors; 2018 ENT Ear Nose & Throat Journal; 97(6).
View source version on businesswire.com: https://www.businesswire.com/news/home/20230713117673/en/
Source: Aerin Medical Inc.
Smart Multimedia Gallery
Two-year data published online in Laryngoscope Investigative Otolaryngology showed a statistically significant and sustained improvement in the symptom burden of those with nasal airway obstruction following a single treatment with VivAer®. A non-invasive technology developed by Aerin Medical Inc., VivAer uses patented, temperature-controlled radiofrequency energy to provide long-term relief from nasal obstruction. (Photo: Business Wire)