Akston Biosciences Corporation, a developer of new classes of biologic therapeutics, announced today it dosed the first set of volunteers in an open-label study of AKS-452, its protein subunit COVID-19 vaccine, as a booster.
- Up to 600 volunteers will participate in an AKS-452 COVID booster study in the Netherlands to investigate boosting the immune response of those previously vaccinated with EMA-registered vaccines
- AKS-452 has shown the ability to successfully neutralize the omicron variant of the SARS-CoV-2 virus
- Shelf stable at 25° Celsius (77° Fahrenheit) for at least 6 months, the low-cost SARS-CoV-2 vaccine can be efficiently manufactured anywhere in the world using standard antibody production facilities
- AKS-452 is currently undergoing Phase II/III clinical testing in India as a primary vaccine; submission for Emergency Use Authorization (EUA) expected by Q3 2022.
BEVERLY, Mass.--(BUSINESS WIRE)-- Akston Biosciences Corporation, a developer of new classes of biologic therapeutics, announced today it dosed the first set of volunteers in an open-label study of AKS-452, its protein subunit COVID-19 vaccine, as a booster. The Phase II booster study is designed to investigate the response of the immune system in up to 600 volunteers who have previously been vaccinated with EMA-registered vaccines from Pfizer, Moderna, Johnson & Johnson (Janssen) and AstraZeneca.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20220511006129/en/
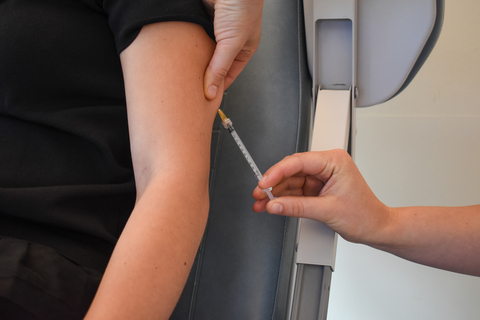
The first volunteer receives Akston Bioscience’s COVID-19 booster vaccine, AKS-452, as part of a Phase II trial in the Netherlands. The protein-based vaccine has been shown to have the ability to successfully neutralize the omicron variant of the SARS-CoV-2 virus, and is shelf-stable for at least six months at room temperatures up to 25°C (77°F). (Photo: Business Wire)
The study is being conducted at the University Medical Center Groningen (UMCG), one of the largest hospitals in the Netherlands. Schelto Kruijff, M.D., Ph.D., the principal investigator at the UMCG for the AKS-452 Phase I and Phase II primary vaccine trials, is serving in the same capacity for the booster trial.
“AKS-452 induced a Th1/Th2 mixed immune response against the Receptor Binding Domain (RBD) of the coronavirus spike protein, according to an analysis of the Phase II study of AKS-452 as a primary vaccine,” Dr. Kruijff said. “While we still need to vaccinate millions of people with a primary COVID-19 shot, it’s also clear that we’ll need an annual booster shot, and key characteristics of AKS-452, including low price and shelf stability, could make AKS-452 an ideal solution.”
The participants, healthy adults between the ages of 18 and 85, will have had their last COVID-19 vaccine shot at least three months earlier and will not have received a COVID-19 booster shot. To determine the efficacy of the AKS-452 as a booster, the study will divide participants into four groups, each of up to 150 subjects, based on having previously received the Pfizer, Moderna, Johnson & Johnson or AstraZeneca vaccine. Each participant will receive one dose of the AKS-452 antigen (90 µg). Antibody levels, virus neutralization activity, and T cell response will be measured over nine months from samples taken in several follow-on appointments to determine the effect over time. The primary efficacy endpoint will be the antibody titer level four weeks after receiving the booster.
The dosing of all volunteers is expected by mid-June. The trial is managed by TRACER Europe B.V., a CRO specializing in fast-track clinical trials and which conducted the Phase I and II trials for AKS-452.
“Because immunity to COVID-19 wanes over time, and because new variants arise, the world will need an ongoing supply of booster vaccines. AKS-452 is cost-effective to manufacture at scale, and does not require the cold-chain necessary for mRNA vaccines. We believe AKS-452 is a practical solution to provide necessary protection, especially for difficult-to-reach populations,” said Todd Zion, Ph.D., President & CEO of Akston Biosciences.
A 1,600 subject Phase II/III clinical trial of AKS-452 as a two-dose primary vaccine is currently underway in India.
About University Medical Center Groningen
The University Medical Center Groningen (UMCG) is one of the largest hospitals in the Netherlands. The more than 12,000 employees work together on care, research, training and education with the common goal: building the future of health. Through innovative and sound research UMCG aims to understand mechanisms of disease; to push borders for diagnostics and treatment; and to help build a network for sustainable health. All of its research focuses on Healthy Ageing. Talent development and state-of-the-art infrastructure are of paramount importance to UMCG.
About TRACER
TRACER Europe B.V. (“TRACER”) is a Clinical Research Organization (CRO) specializing in fast-track solutions for testing innovative biologic medicines and is a founding member of the COVID-19 Rapid Cure Task Force (RCTF). TRACER and its partners provide its clients with the expertise, infrastructure, and capacity to rapidly generate accurate first-in-human clinical data. www.tracercro.com.
About Akston Biosciences
Akston Biosciences Corporation leverages its novel fusion protein platform to develop and manufacture new classes of biologics, including vaccines, ultra-long-acting insulins, and autoimmune disease therapies. Founded by the team that developed the world’s first clinical glucose-responsive insulin at SmartCells, Inc. (sold to Merck & Co.), Akston has partnered with Dechra Pharmaceuticals PLC to commercialize once-a-week canine and feline insulin therapies. It operates a GMP biologics manufacturing facility and research laboratory at its Beverly, Mass. location. www.akstonbio.com.
View source version on businesswire.com: https://www.businesswire.com/news/home/20220511006129/en/
Contacts
Linda Pendergast-Savage
Birnbach Communications for Akston Biosciences
+1-508-224-7905
lpendergastsavage@birnbachcom.com
Source: Akston Biosciences Corporation
Smart Multimedia Gallery
The first volunteer receives Akston Bioscience’s COVID-19 booster vaccine, AKS-452, as part of a Phase II trial in the Netherlands. The protein-based vaccine has been shown to have the ability to successfully neutralize the omicron variant of the SARS-CoV-2 virus, and is shelf-stable for at least six months at room temperatures up to 25°C (77°F). (Photo: Business Wire)