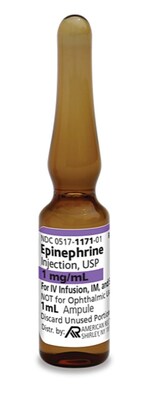
American Regent announces the launch of sulfite-free Epinephrine Injection, USP.
|
MELVILLE, N.Y., March 30, 2023 /PRNewswire/ -- American Regent, Inc. announces the launch of sulfite-free Epinephrine Injection, USP.1 Epinephrine Injection, USP, is indicated for emergency treatment of allergic reactions (Type I), including anaphylaxis, which may result from insect stings or bites, foods, drugs, sera, diagnostic testing substances and other allergens, as well as idiopathic anaphylaxis or exercise-induced anaphylaxis. Epinephrine is also indicated to increase mean arterial blood pressure in adult patients with hypotension associated with septic shock. "With the approval of this generic alternative we continue to show our commitment to ensuring both patients and providers have access to quality treatment options," stated Joann Gioia, Vice President and Chief Commercial Officer at American Regent, Inc. Product is available for immediate shipment. Customers can order Epinephrine Injection, USP through their wholesaler/distributor or by contacting our Customer Support Group at 1-800-645-1706. Epinephrine Injection, USP is supplied as follows:
See the following Important Safety Information in addition to the Full Prescribing Information. For additional information, please visit americanregent.com. Reference: Epinephrine Injection, USP For intramuscular, subcutaneous, and intravenous use. INDICATIONS AND USAGE Anaphylaxis Hypotension associated with Septic Shock IMPORTANT SAFETY INFORMATION DOSAGE AND ADMINISTRATION: See Full Prescribing Information for instructions on dilution and administration of the injection. CONTRAINDICATIONS: None. WARNINGS AND PRECAUTIONS Do not inject into buttock, digits, hands, or feet. Extravasation and Tissue Necrosis with Intravenous Infusion: Avoid extravasation into tissues, which can cause local necrosis. When epinephrine is administered intravenously, check the infusion site frequently. May aggravate angina pectoris or produce ventricular arrhythmias. Serious Infections at the Injection Site: Rare cases of serious skin and soft tissue infections, including necrotizing fasciitis and myonecrosis have been reported. Hypertension: Because individual response to epinephrine may vary significantly, monitor blood pressure frequently and titrate to avoid excessive increases in blood pressure. Patients receiving monoamine oxidase inhibitors or antidepressants of the triptyline or imipramine types may experience severe, prolonged hypertension. Pulmonary Edema: Epinephrine increases cardiac output and causes peripheral vasoconstriction, which may result in pulmonary edema. Renal Impairment: Epinephrine constricts renal blood vessels, which may result in oliguria or renal impairment. Cardiac Arrhythmias and Ischemia: Epinephrine may induce cardiac arrhythmias and myocardial ischemia, especially patients suffering from coronary artery disease, or cardiomyopathy. Allergic Reactions Associated with Sulfite—This product does not contain sodium bisulfite. ADVERSE REACTIONS: Common adverse reactions to systemically administered epinephrine include, but are not limited to, anxiety, apprehensiveness, restlessness, tremor, weakness, dizziness, sweating, palpitations, pallor, nausea and vomiting, headache, and respiratory difficulties. These symptoms occur in some persons receiving therapeutic doses of epinephrine, but are more likely to occur in patients with heart disease, hypertension, or hyperthyroidism. Patients with Parkinson's disease may experience psychomotor agitation or a temporary worsening of symptoms. Diabetic patients may experience transient increases in blood sugar. USE IN SPECIFIC POPULATIONS Pregnancy Labor or Delivery: There are risks to the mother and fetus associated with epinephrine use during labor or delivery. Avoid epinephrine during the second stage of labor. Although epinephrine may improve maternal hypotension associated with septic shock and anaphylaxis, it may result in uterine vasoconstriction, decreased uterine blood flow, and fetal anoxia. Lactation Pediatric Use Safety and effectiveness of epinephrine in pediatric patients with septic shock have not been established. Geriatric Use OVERDOSAGE: For additional Safety Information, please see Full Prescribing Information. You are encouraged to report Adverse Drug Events (ADEs) to American Regent, Inc. at 1.800.734.9236 or to the FDA by visiting www.fda.gov/medwatch or calling 1.800.FDA.1088. REF-0848 5/2022 You are encouraged to report adverse drug events (ADEs) to American Regent: ADEs may also be reported to the FDA: Medical information: For medical information outside of normal business hours About American Regent American Regent is committed to US-based manufacturing. To that end, over the last several years, we have made significant investments in expanding and modernizing our manufacturing facilities in Ohio and New York. This expansion will nearly double our capacity and allow us to better serve our customers now and in the future. Speed counts. Flexibility matters. Reliability and quality are paramount. Because patients should never have to wait for the medications, they need. For more information, please visit www.americanregent.com. About Daiichi Sankyo For more information, please visit: www.daiichisankyo.com.
SOURCE American Regent, Inc. |