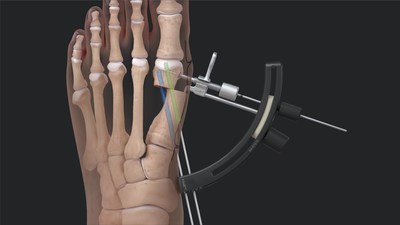
Arthrex, a global leader in minimally invasive orthopedic technology, has launched the award-winning Arthrex Minimally Invasive Bunionectomy System to offer patients a better solution to traditional bunion surgery.
|
Arthrex Bunionectomy offers better results with faster recovery than traditional surgery NAPLES, Fla., Dec. 5, 2022 /PRNewswire/ -- Arthrex, a global leader in minimally invasive orthopedic technology, has launched the award-winning Arthrex Minimally Invasive Bunionectomy System to offer patients a better solution to traditional bunion surgery. Arthrex Bunionectomy offers better results with faster recovery than traditional surgery
The Arthrex Bunionectomy System is designed to be an all-inclusive set to facilitate minimally invasive surgical bunion correction. It is clinically proven to achieve the same or better corrective results than traditional surgery with less downtime, pain and swelling.1,2 Performed through tiny incisions, this minimally invasive procedure means patients can achieve complete bunion correction and get back on their feet faster. Studies show Arthrex Bunionectomy patients' average recovery is up to eight weeks faster than traditional open procedures, as published in the journal Foot & Ankle International.2 Patients also experienced more cosmetically appealing results, with smaller incisions that leave behind almost no visible scars.2 The Arthrex Minimally Invasive Bunionectomy System earned a 2022 Edison Award in the Medical Treatment category. The Edison Awards honor excellence in new product and service development, marketing, human-centered design and innovation. Along with the procedure, Arthrex launched two websites geared toward patients and surgeons, respectively. BunionPain.com offers patients educational materials and connects them with local surgeons trained in the Arthrex Bunionectomy. Bunionectomy.arthrex.com is geared toward clinicians and offers a way to connect with Arthrex Technology Consultants and Medical Education representatives for information on and training in the Arthrex Bunionectomy System. Users can also download a guide with further information and a list of suggested questions once they're ready to speak to a provider. About Arthrex
Arthrex Inc., headquartered in Naples, Florida, is a global leader in multi-specialty, minimally invasive surgical technology, medical research, manufacturing and medical education. Arthrex develops and releases more than 1,000 new products and procedures every year to advance minimally invasive orthopedics, trauma, spine and arthroplasty innovation worldwide, and specializes in the latest 4K multi-specialty surgical visualization and OR integration technology solutions. For more information, visit www.arthrex.com. References
SOURCE Arthrex, Inc. |