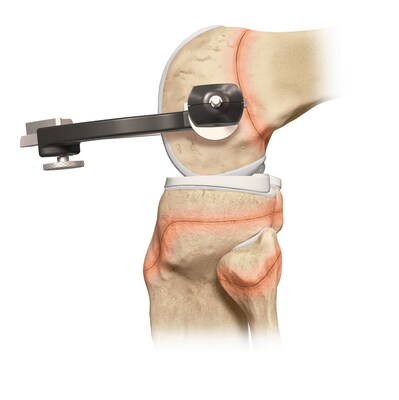
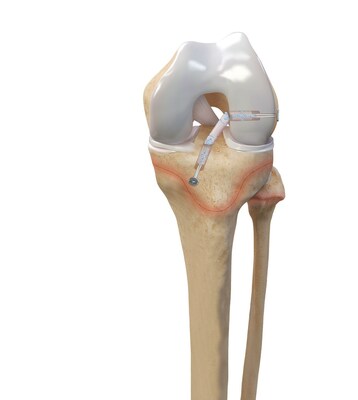
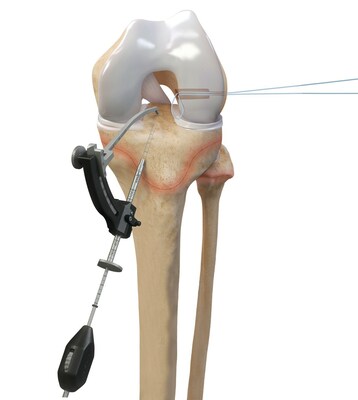
Arthrex announced today its ACL TightRope implant has received clearance from the U.S. Food and Drug Administration (FDA) for pediatric indications.
|
Surgical instruments, implants developed for pediatric, young adolescent indications in partnership with top surgeons from Hospital for Special Surgery NAPLES, Fla., Jan. 10, 2023 /PRNewswire/ -- Arthrex, Inc., a global leader in minimally invasive surgical technology, announced today its ACL TightRope implant has received clearance from the U.S. Food and Drug Administration (FDA) for pediatric indications. The TightRope implant is used in the surgical treatment of orthopedic injuries and is the first and only fixation device for anterior cruciate ligament (ACL) injuries cleared for pediatric use. "This is a significant achievement in orthopedic surgery." -Arthrex President and Founder Reinhold Schmieding
Arthrex developed all-epiphyseal and transphyseal techniques and instrumentation for ACL surgery alongside top orthopedic surgeons Frank A. Cordasco, MD, MS, and Daniel W. Green, MD, MS, FAAP, FACS, from the Hospital for Special Surgery (HSS), one of the world's leading academic medical centers focused on musculoskeletal health. HSS is the top-ranked hospital in the U.S. for orthopedics for the 13th consecutive year and is top ranked in pediatric orthopedics by U.S. News and World Report.1 "For more than a decade, Arthrex has worked closely with leading orthopedic surgeons from HSS to develop minimally invasive solutions for pediatric and young adolescent ACL surgery," said Arthrex President and Founder Reinhold Schmieding. "We are proud to partner with surgeons from the Hospital for Special Surgery to design treatment options specifically for ACL injuries in younger patients. This is a significant achievement in orthopedic surgery and another testament to Arthrex's dedication to its mission of Helping Surgeons Treat Their Patients Better™, starting from an early age." Drs. Cordasco and Green helped Arthrex develop pediatric and young adolescent-specific instrumentation guides to address a growing population of young athletes facing ACL injuries. "The expansion of Arthrex's knee ligament portfolio to include pediatric- and young-adolescent-specific instrumentation and implants, along with the new indications cleared by the FDA, represents a substantial improvement on existing treatment options in this high-risk population of athletes," said Dr. Cordasco. While ACL injuries are most common in patients between the ages of 10 and 29, previous treatment options for the younger end of this patient population have centered on adapting surgical techniques and devices initially developed for adults. The Arthrex all-epiphyseal technique was developed for skeletally immature patients and involves avoiding the pediatric growth plates to repair or reconstruct the ACL. With the pediatric-specific instrumentation guides, surgeons drill sockets for the new, reconstructed ACL by avoiding the growth plate to diminish the potential for growth disturbance. In older adolescent patients who are approaching skeletal maturation, surgeons will drill across the growth plates to reconstruct the ACL using an all-soft-tissue autograft. Of the two all-soft-tissue autografts available for use in this young athlete population, quadriceps autografts have been found to have superior outcomes compared to hamstring autografts with lower revision rates and greater return-to-sport rates.2 "These pediatric- and young-adolescent-specific guides help surgeons address the young athlete's unique anatomy, greatly enhancing surgical options for reconstruction and epiphyseal fixation for ligamentous reconstruction or avulsion injury repair," said Dr. Green. ACL injuries are one of the most common sports injuries, with more than 200,000 occurring each year.3 Injuries occur predominantly in the young and sports-active population and are typically caused by non-contact twisting or hyperextension injuries but can also occur due to contact injuries. The Arthrex ACL TightRope portfolio of fixation devices includes the ACL TightRope II implant, the TightRope attachable button system (ABS) and implant, the FiberTag® TightRope implant and the ACL Repair TightRope implant with FiberRing™ sutures. For more information, visit https://www.arthrex.com/knee/acl-tightrope-ii-implant. About Arthrex
Arthrex Inc., headquartered in Naples, Florida, is a global leader in multispecialty, minimally invasive surgical technology, medical research, manufacturing and medical education. Arthrex develops and releases more than 1,000 new products and procedures every year to advance minimally invasive orthopedics, trauma, spine and arthroplasty innovation worldwide and specializes in the latest 4K multi-specialty surgical visualization and OR integration technology solutions. For more information, visit www.arthrex.com. References
SOURCE Arthrex, Inc. |