Biopharma and life sciences companies from across the globe provide updates on their businesses and pipelines.
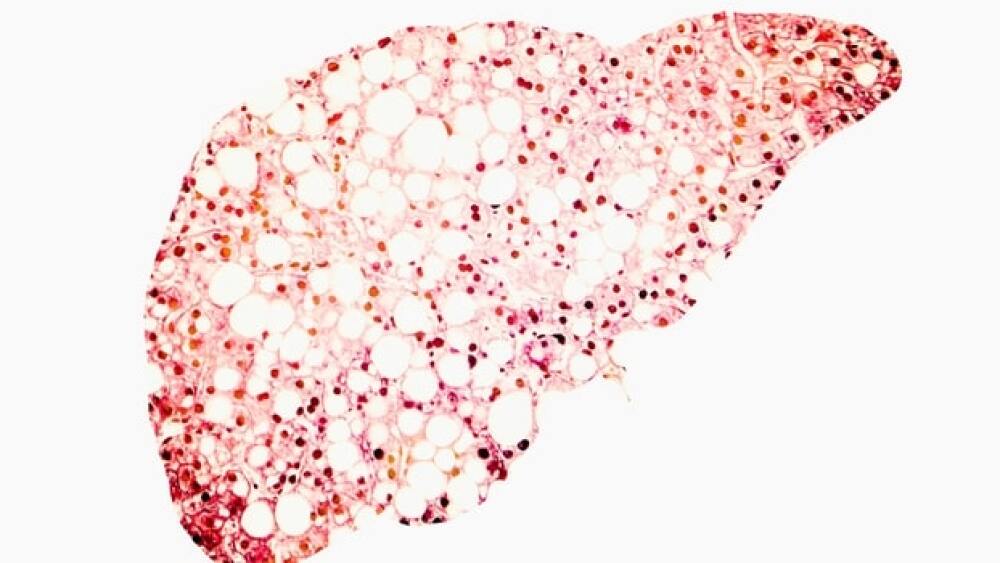
Nonalcoholic steatohepatitis (NASH) has been a difficult nut to crack for the pharmaceutical industry. Multiple therapeutic programs have fallen short over the past few years. However, this week, France-based GENFIT launched NASHnext, a novel, non-invasive diagnostic test for NASH powered by the company’s proprietary diagnostic technology, NIS4. The test is offered in the U.S. through Labcorp.
According to GENFIT, NASHnext can identify NASH and significant fibrosis in patients with at least one metabolic risk factor. Data supporting this claim was published in The Lancet Gastroenterology and Hepatology.
NASH affects more than 16 million people across the United States and about 80 million across the globe. The number of patients is expected to increase over the next few years. It is the most severe form of nonalcoholic fatty liver disease. It is estimated that only 5% of patients are aware of their disease, primarily due to the asymptomatic nature of NAFLD and limited availability of tests. NASHnext is a blood-based test capable of diagnosing NASH and significant to advanced liver fibrosis without relying on an invasive liver biopsy.
Elsewhere across the globe:
Sobi – Swedish Orphan Biovitrum AB and the Hellenic Institute for the Study of Sepsis today announced positive top line results from the SAVE-MORE study that assessed the effect of anakinra in moderate to severe COVID-19 pneumonia patients. Data showed that early and targeted use of anakinra in addition to current standard of care in hospitalized patients with poor prognosis prevented either death or progression to severe respiratory failure, whilst increasing the number of patients who were discharged from hospital with no evidence of COVID-19 infection. At day 28, patients treated with anakinra in addition to current standard of care saw significant improvement. There were reductions in the number of patients who died or who progressed to severe respiratory failure, as well as an increase in the number of patients who were discharged from hospital with no evidence of COVID-19 infection, Sobi said.
HUTCHMED – Formerly known as Chi-Med, Hong Kong-based HUTCHMED completed its first New Drug Application for the U.S. Food and Drug Administration for the oncology drug surufatinib for the treatment of pancreatic and extra-pancreatic neuroendocrine tumors (NETs). The NDA is supported by data from two Phase III studies of NETs. The FDA granted Fast Track designation to surufatinib in 2020, which set the stage for the NDA on a rolling basis. In addition to Fast Track designation, the FDA also granted Orphan Drug designation to surufatinib for pancreatic NET.
Mogrify Limited – U.K.-based Mogrify announced the second closing of $17 million in a Series A financing round. Combined with a previous raise, this brings the company’s total Series A to $33 million. Funding will be used to support the advancement of Mogrify’s immuno-oncology and ophthalmology programs, as well as continued platform development and the exploration of cell reprogramming for novel therapeutic application. This second close of the Company’s Series A was led by Parkwalk Advisors and incorporates additional funding from strategic Corporate Pharma investor Astellas Venture Management as well as 24Haymarket, co-Founder of Abcam PLC, Jonathan Milner, and Mogrify CEO Darrin M Disley.
Relief Therapeutics – Switzerland’s RELIEF Therapeutics is acquiring all outstanding shares of APR Applied Pharma Research S.A., a privately-held Swiss pharmaceutical company. APR harnesses proprietary delivery systems and novel dosage forms to optimize the therapeutic potential of pharmaceuticals and improve patient outcomes. APR’s pipeline include products for the treatment of rare or debilitating diseases, including Golike, a therapy to improve metabolic control in patients suffering from phenylketonuria, a rare genetic metabolic disorder. In addition to Golike, APR received clearance as a class III medical device in Europe for Sentinox, an intranasal spray to help block the transmission of the SARS-CoV-2 virus.
Capitainer – Swedish medtech startup Capitainer announced its home self-sampling kit for analysis of COVID-19 antibody levels, has been shown to be suitable for clinical population testing. In a large scale population study conducted in Sweden, Capitainer said 97% of the samples self-collected and returned to the lab met the official quality standard for accurate analysis resulting in clinical diagnosis.
Solentim -- U.K.-based Solentim announced an expansion of its European operations to bolster its workflows for antibody and cell-based therapies. The company has established a new EU entity based in Ireland to enable “frictionless business” within the European Union. Additionally, Solentim opened a new warehousing and logistics hub in Germany, to better serve its customers within the EU. CEO Mark Truesdale said the European market is one of the fastest-growing parts of its business. The new facilities will allow the company to better serve that fast-growing part of the company’s business, Truesdale said.