In announcing the approval this morning, Janssen said IL-23 is a naturally occurring cytokine involved in normal inflammatory and immune responses associated with symptoms of psoriatic arthritis.
The U.S. Food and Drug Administration (FDA) approved Janssen Pharmaceutical‘s Tremfya as a treatment for active psoriatic arthritis. The approval marks the first time a selective interleukin (IL)-23 inhibitor has been approved as a treatment for the chronic progressive disease.
In announcing the approval this morning, Janssen said IL-23 is a naturally occurring cytokine involved in normal inflammatory and immune responses associated with symptoms of psoriatic arthritis. In the clinical studies that led to the approval of Tremfya in this indication, the IL-23 inhibitor was shown to relieve patients’ pain in their soft tissue and inflammation in their fingers and toes. An analysis of data from two studies, DISCOVER-1 and DISCOVER-2, Tremfya taken every eight weeks resolved inflammation of the entheses, the points where ligaments and tendons attach to the bone, in 50% of patients, compared to 29% for placebo. Also, Tremfya resolved dactylitis, inflammation in the joints of fingers and toes, in 59% of patients, versus 42% receiving placebo. In addition to improving symptoms of psoriatic arthritis in joints, Tremfya improved skin manifestations due to the disease in those patients who had psoriatic skin involvement, Janssen said.
In the clinical trials, Tremfya hit the American College of Rheumatology 20% (ACR20) improvement mark at 24 weeks, with 52% and 64% of patients achieving an ACR20 response compared to 22% and 33% in patients treated with placebo, respectively.
Janssen’s DISCOVER program includes the first-ever Phase III studies evaluating an IL-23 p19 inhibitor for the treatment of psoriatic arthritis, the company said. Tremfya, a human monoclonal antibody that selectively blocks the p19 subunit of interleukin (IL)-23, has already been approved for the treatment of adult patients with moderate to severe plaque psoriasis who may benefit from systemic therapy or phototherapy.
David M. Lee, Therapeutic Area Head of Immunology at Janssen Research and Development, said Tremfya is the only selective IL-23 inhibitor approved for both active psoriatic arthritis and moderate to severe plaque psoriasis. It is also the only biologic that has been approved for psoriatic arthritis that has been shown to improve fatigue as measured by FACIT-F, he said.
“At Janssen, we strive to reimagine what is possible in how immune-mediated diseases like active psoriatic arthritis are understood and treated. Today’s approval marks an exciting milestone as we follow the science and search for solutions for patients with these complicated diseases,” Lee said in a statement.
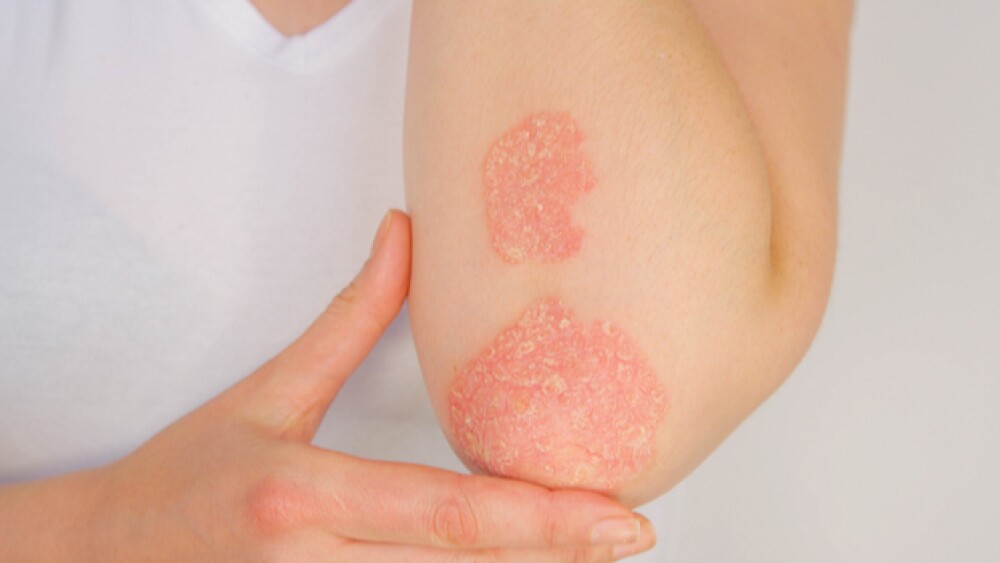
Psoriatic arthritis is a chronic, immune-mediated inflammatory disease characterized by joint inflammation, enthesitis, dactylitis and the skin lesions associated with psoriasis. It is estimated that at least one million Americans are living with psoriatic arthritis and up to 30% of patients living with psoriasis can develop psoriatic arthritis. The disease causes pain, stiffness and swelling in and around the joints.