The U.S. FDA has released a Complete Response Letter (CRL) following its review of LEO Pharma’s tralokinumab. Further data on a device component is the issue brought to light by the recently completed Biologics License Application (BLA) review.
The U.S. Food and Drug Administration (FDA) has released a Complete Response Letter (CRL) following its review of LEO Pharma’s tralokinumab. Further data on a device component, rather than the safety and efficacy of tralokinumab, is the issue brought to light by the recently completed Biologics License Application (BLA) review.
Jörg Möller, Executive Vice President for Global R and D at LEO Pharma, is quick to assure, “The FDA has not raised any questions to the clinical efficacy or safety of tralokinumab, but only requested additional data relating to a device component of the combination product. We will now work closely with the FDA to address their request and bring tralokinumab to the U.S. patients as quickly as possible.” Moller iterates the pharma’s commitment to put tralokinumab into the hands of those who need it most.
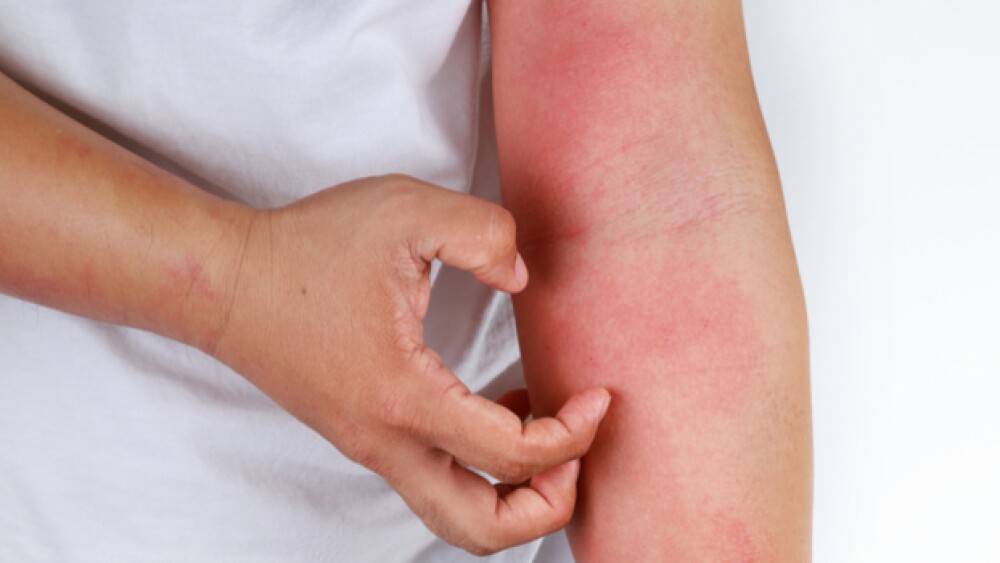
Tralokinumab, or Adtralza®, is the Danish pharmaceutical’s answer to moderate to severe atopic dermatitis (A.D.), also known as atopic eczema. In its uncontrolled moderate and severe state, this disease may have debilitating physical effects and even lead to mental health issues. Key to the inflammation that underpins A.D. are the proteins IL-13 and IL-4.
“Atopic dermatitis is characterized by its unpredictability, which can be challenging for patients who often experience physical discomfort and emotional effects that may continue for decades,” Möller explains.
While A.D. is considered to be heightened, even unusual, immune reaction, it has not necessarily been categorized as an autoimmune disease. Rather, it is an atopic disease.
LEO Pharma’s asset would offer A.D. patients a new way of treating their condition. Tralokinumab targets and neutralizes the IL-13 cytokine. By suppressing this chemical messenger, the biologic effectively prevents an overreaction of the immune system to allergens. This offers a different approach than current treatment options, including topical corticosteroids, topical immunomodulators, and phototherapy.
To date, the FDA has only approved one biologic for the treatment of A.D. Dupilumab, also known as Dupixent, was developed by American biotech companies Regeneron Pharmaceuticals and Sanofi Genzyme. The immunosuppressant drug, while similar to tralokinumab in blocking IL-13, also blocks the IL-4 cytokine. Dupilumab is approved by European Medicines Agency (EMA).
Still, despite the added measures required by the FDA’s CRL in the U.S., tralokinumab is enjoying wins elsewhere, and A.D. patients in the European Union could very well be using the biologic before long. Recently, the Committee for Medicinal Products for Human Use (CHMP) of EMA announced their recommendation that Adtralza® be granted marketing authority.
The CHMP support is significant and will now usher in releasing the European Commission’s decision on the Marketing Authorization Application for the A.D. drug. This would be the last step before tralokinumab begins marketing in the E.U.
CHMP’s opinion is primarily based on data captured through the ECZTRA 1, 2, and ECZTRA 3 clinical trials. These studies verified tralokinumab’s safety as well as efficacy. Results were gauged against the Investigator Global Assessment score (IGA 0/1) score and the Eczema Area and Severity Index score (EASI-75). The latter indicated improvement in the area of 75% among the participants.