How do you catch just one kind of fish in a sea of similar fish? That’s the question being answered by researchers at Nurix, except they aren’t catching fish – they are ‘catching’ proteins in your body.
How do you catch just one kind of fish in a sea of similar fish? That’s the question being answered by researchers at Nurix, except they aren’t catching fish – they are ‘catching’ proteins in your body. Nurix is developing drugs that can specifically target one protein within the hundreds or thousands of extremely similar proteins in the body, ultimately enabling them to regulate hard-to-drug or ‘undruggable’ proteins.
Mutated or dysregulated proteins cause or contribute to a vast majority of diseases, including cancers and neurodegenerative diseases like Alzheimer’s. Normally, levels of all the proteins in your body are carefully balanced by constantly making and destroying proteins. Protein levels can build up if too much is made or not enough is destroyed and vice versa (protein levels can drop if not enough is made or too much is destroyed).
In diseases where too much protein is around, especially if the protein is mutated or dysfunctional, getting rid of that harmful protein by degrading it could treat the disease. This is where Nurix comes in.
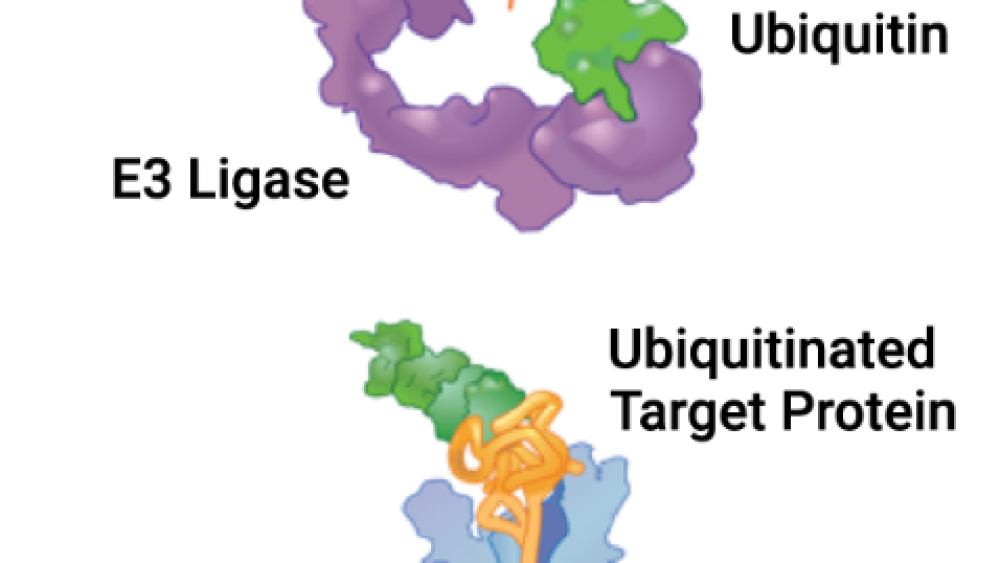
Nurix has two platforms that control how much a specific protein is degraded in the body: one to increase and one to decrease specific protein degradation. Both platforms take advantage of the body’s own protein waste removal system, called the ubiquitin proteasome system (UPS, shown in the picture). Ubiquitin (shown in green) is a small protein that acts as a ‘molecular tag’ by tagging protein waste (binding to old or misfolded proteins) for removal. Ubiquitin attachment relies upon the sequential activities of proteins in the UPS, from activating enzymes (E1) to conjugating enzymes (E2) and finally ligases (E3). Ubiquitin-tagged proteins are taken to the cellular garbage disposal, a protein complex called the proteasome (shown in blue).
E3 ligases (shown in purple) are the key pathway components because they directly attach ubiquitin to the target protein (shown in gold) in the final step of the UPS; they are the gatekeeper between a protein being destroyed or not. Therefore, controlling the E3 ligase for a certain protein allows researchers to directly control the fate of the protein, either forcing it to be degraded or preventing its degradation.
“We focused on E3 ligases because they confer control and specificity to the UPS,” said Arthur Sands, CEO of Nurix. “They are key to regulating the natural machinery that could restore protein homeostasis when a disease-causing protein is out of control, such as a mutated protein in cancer.”
The beauty of this approach is that it can theoretically target any protein (even the ‘undruggable’ ones) because it doesn’t rely on the target protein having a high-affinity binding pocket (called an active site) as typical enzymatic inhibitors do; these small molecule drugs will bind to any compatible binding site on the target protein. It would also (theoretically) be harder for resistance to develop against drugs that utilize the protein waste system because the drug can be designed to interact with a ligase that is essential to cell viability, not just the target protein.
Nurix recently presented new data on its Bruton Tyrosine Kinase (BTK) degrader platform at the American Society of Hematology (ASH) annual meeting on December 7th. Before we dive into the new data, let’s get a better understanding of how their protein degrader platform works and how it can treat diseases.
Nurix’s targeted protein modulation platform
E3 ligases are hard to specifically target because of their complexity (they function in multiprotein complexes, not just one protein) and sheer number – there are over 700 in the human body! Similar to kinases with their specific substrates, each E3 ligase has its own set of proteins that it can tag for disposal, making them attractive targets for specific (and therefore less toxic) therapies. Unlike kinases, however, developing drugs against E3 ligases has proven to be difficult, resulting in very limited success, leading many researchers to historically deem E3 ligases ‘undruggable.’
“Nurix’s lead immuno-oncology program is a small molecule inhibitor of CBL-B, an E3 ligase that suppresses T-cell activation,” said Sands. “Our CBL-B inhibitor illustrates the arm of our platform that allows us to increase specific protein levels by blocking the normal protein degrading activity of a particular ligase. This small molecule drug candidate is a potent stimulator of T-cell function, including IL-2 secretion, that can be dosed orally and may offer certain advantages over current antibody therapies in the space.”
Although Nurix has made the biochemical activity of certain E3 ligases screenable, it has also enabled E3 ligases for direct binding screens using DNA-encoded libraries (DELs). Using DELs allows them to create and screen huge chemical libraries (including about 1 billion proprietary compounds) to find and build small molecules that can bind both the target protein and its E3 ligase (from the 30 ligases Nurix screens against). They can even identify E3 ligase-specific drugs that can kill cancer cells resistant to other drugs.
“Our library was developed and built to include custom scaffolds with drug-like physical properties to identify binders with a range of affinities for E3 ligases and new protein targets,” Sands explained. “This allows our medicinal chemistry team to design small molecule drug candidates that are attractive for therapeutic purposes with oral dosing and good bioavailability.”
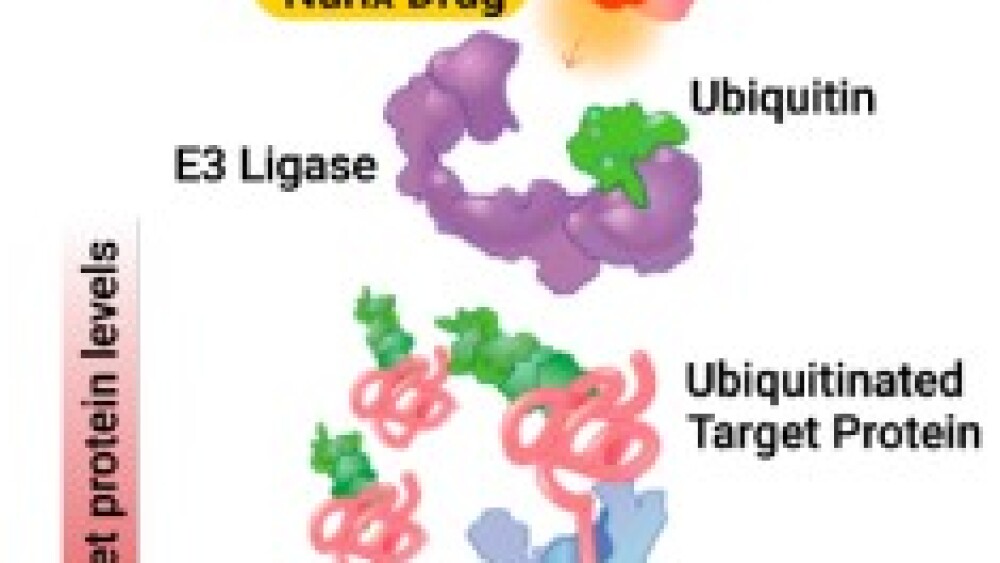
The other side of Nurix’s platform, called its ‘degrader’ platform, uses a small molecule drug (highlighted in gold in the picture) to direct the target protein (in pink) to its E3 ligase (in purple). This enables the E3 ligase to add a ubiquitin tag (in green) to the protein. The tagged protein is then sent to the proteasome (in blue) for degradation.
Therefore, the drug, which Nurix calls a Chimeric Targeting Molecule (CTM), harnesses the body’s own protein waste removal system to reduce levels of the undesirable target protein.
“Targeting the ubiquitin proteasome pathway is a powerful method to degrade the target protein rather than inhibiting it,” added Sands. “Drugs targeting this pathway are potent and catalytic because one E3 ligase can affect thousands of target proteins, rather than the traditional one-to-one ratio needed for protein inhibitors. Ideally, a patient would only need a small amount of the drug to see the desirable effects with fewer side effects.”
Essentially, Nurix’s small molecule drugs are PROTACs (proteolysis-targeting chimeras), which are small molecules that act as ‘molecular glue’ by linking a target protein to an E3 ligase to facilitate ubiquitinylation. PROTACs have three parts: one end that binds to the target protein, the other end that binds to the E3 ligase, and a linker connecting the two ends.
Although PROTACs were only discovered in 2001, they have become quite a hot topic recently due to their potential to target hard-to-drug or currently ‘undruggable’ proteins. Not surprisingly, quite a few other companies trying to harness the potential of PROTACs in various diseases, such as Arvinas in metastatic breast or prostate cancer (in Phase 1 trials, the first in the clinic) and Kymera Therapeutics in various cancers and inflammatory diseases (in preclinical studies). There are also a handful of other companies developing drugs to modulate protein degradation in other ways, including Cedilla Therapeutics, C4 Therapeutics, and Cullgen.
New data presented at ASH
Although Nurix is still a preclinical company, they talked to BioSpace about new data they recently presented at ASH on their Bruton tyrosine kinase (BTK) degrader molecule NRX0492.
BTK is an essential protein involved in the development and maturation of B-cells, specialized white blood cells that are part of the immune system. BTK is also crucial for B-cell signaling and communication, so much so that some B-cells depend on BTK signaling for their survival.
However, dysfunctional BTK proteins and signaling play a crucial role in some B-cell malignancies, such as chronic lymphocytic leukemia (CLL). Therefore, inhibiting aberrant BTK signaling is a therapeutic strategy for those B-cell lymphomas. There are currently three FDA-approved BTK inhibitors: ibrutinib (Imbruvica) approved in 2013, acalabrutinib (Calquence) approved in 2017, and zanubrutinib (Brukinsa) approved in 2019.
Long-term treatment with BTK inhibitors is currently used to treat certain B-cell cancers; however, the long-term nature of the treatment can lead to drug resistance if tumor cells mutate to reactivate BTK.
Nurix aims to navigate around this BTK inhibitor resistance using its small molecule NRX0492 to facilitate BTK’s degradation rather than its inhibition. Focusing on the natural protein removal system to get rid of the protein (therefore reducing its signaling) offers a route around any drug resistance that may arise.
“NRX0492 combines two molecules: a ‘hook’ that binds BTK with a ‘harness’ that recruits ubiquitin ligases that mediate proteasome-dependent degradation of BTK,” Sands told BioSpace. “In vitro studies in human tumor cells (ABC-DLBCL cell line, TMD8 and primary human CLL cells), and in vivo studies (oral administration in mice) show that subnanomolar concentrations of NRX0492 are highly effective at degrading BTK with rapid onset and sustained activity after drug washout.”
Importantly, NRX0492 was not only effective at degrading normal BTK, but it could also effectively degrade ibrutinib-resistant BTK. “This highlights NRX0492’s therapeutic potential for CLL patients with ibrutinib resistance,” Sands added. “Our collaborators at NIH also presented data with NRX0492 that showed it dramatically slowed growth of patient’s CLL cells in mouse PDX models with oral dosing.”
The BTK drug development space is gaining traction, especially with the recent news of Merck acquiring ArQule, a biotech company who’s lead compound (ARQ 531) is a BTK inhibitor in Phase 2 studies for B-cell malignancies.
As there are only two protein degrading drugs being tested in Phase 1 clinical trials currently, only time will tell how they fair in terms of safety and efficacy. Nurix aims to be in the clinic in late 2020 with a BTK-CTM small molecule degrader similar to NRX0492 as their first clinical program.