SpringWorks Therapeutics and BeiGene, Ltd. have teamed up to develop therapeutics that will target advanced solid tumors that contain RAS mutations, as well as other MAPK aberrations.
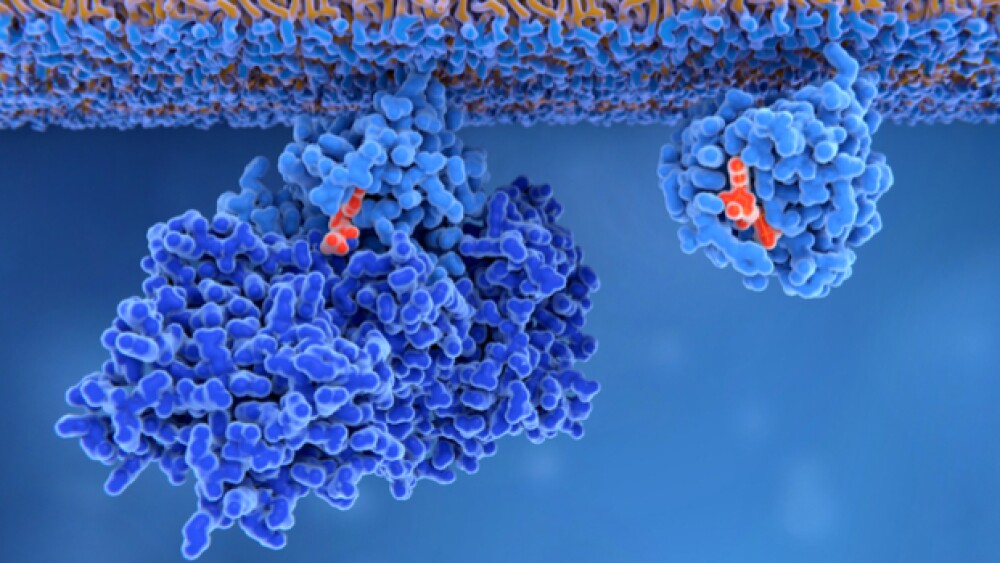
Membrane-bound Ras protein in its inactive form (left) and activated form (right). Mutations in this protein can lead to solid tumors.
SpringWorks Therapeutics and BeiGene Ltd have teamed up to develop therapeutics that will target advanced solid tumors that contain RAS mutations, as well as other MAPK aberrations.
The two companies will combine BeiGene’s investigational RAF dimer inhibitor, lifirafenib (BGB-283) and SpringWorks Therapeutics’ investigational MEK inhibitor, PD-0325901 in patients with advanced solid tumors. BeiGene will be responsible for administering the Phase 1b clinical trial, which is expected to begin in the first quarter of 2019, the companies said this morning. While BeiGene will oversee the trial, both companies will share the costs. SpringWorks Therapeutics will also oversee fixed-dose formulation work as part of the collaboration.
Preclinical models have shown the potential efficacy of combining MEK and RAF inhibition in RAS-mutant solid tumors. By vertically inhibiting the MAPK pathway, the combination approach of PD-0325901 and lifirafenib may potentially overcome feedback loops that have impeded therapeutic development for RAS-mutant solid tumors, the companies announced this morning.
BeiGene’s lifirafenib is an investigational small molecule kinase inhibitor with RAF monomer and dimer inhibition activities. The company said Lifirafenib has shown antitumor activities in preclinical models and in cancer patients with tumors harboring BRAF V600E mutations, non-V600E BRAF mutations, non-small cell lung cancer and endometrial cancer harboring KRAS mutations. The investigational drug has been dosed in 150 patients, BeiGene said.
John Oyler, co-founder and chief executive officer of BeiGene, said the company is excited about exploring the combination of lifirafenib with SpringWorks’ investigational MEK inhibitor for treatment of patients with RAS mutations. Oyler noted that patient population is one with high unmet need.
“Mutations in RAS genes are found in roughly one-fourth of all human cancers, making this a critically important area for developing new cancer treatments. Despite decades of research, no anti-RAS therapies have been approved to date,” said Saqib Islam, CEO of SpringWorks, said in a statement.
Islam, who took over the reins of SpringWorks this week, said the combination of a MEK inhibitor with a RAF dimer inhibitor “has strong scientific rationale.” Islam added that he’s excited about building on the existing preclinical data that has “demonstrated potential benefits with this combination therapy approach.”
Originally discovered by Pfizer, SpringWorks’ PD-0325901 inhibits MEK, a key signaling protein for cellular survival and proliferation. The investigational therapeutics has been shown to block MEK phosphorylation in clinical biopsies. That blocking causes the cells to die.
The announcement comes about a month after BeiGene positive preliminary topline results from its Phase II trial of tislelizumab, the company’s investigational checkpoint inhibitor for relapsed/refractory classical Hodgkin’s lymphoma (R/R cHL). BeiGene said patients on tislelizumab have shown an overall response rate of 73 percent and a complete response rate of 50 percent. BeiGene’s investigational Bruton’s tyrosine kinase (BTK) inhibitor zanubrutinib also snagged Fast Track designation by the U.S. Food and Drug Administration (FDA) for the treatment of patients with Waldenström macroglobulinemia.
In addition to its combination program with BeiGene, SpringWorks said it intends to launch a Phase IIb study of PD-0325901 as monotherapy for neurofibromatosis type 1 patients with plexiform neurofibromas in 2019.