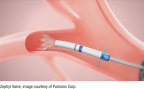
A new publication this month in the Annals of the American Thoracic Society confirms the effectiveness of the Zephyr Valve® in improving dyspnea (shortness of breath), ability to exercise, and quality of life for patients with COPD/emphysema.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20200512005517/en/
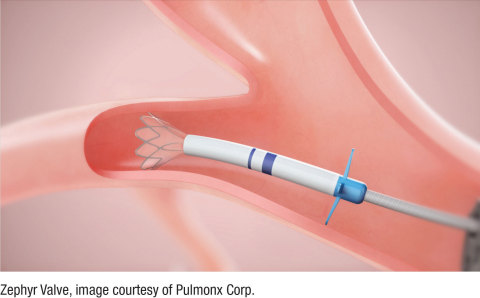
The Zephyr Valve for Emphysema / COPD (Photo: Business Wire)
- Patients with severe emphysema and hyperinflation treated with Zephyr Valve experience “moderate” to “large” improvements in multidimensional scores for breathlessness, activity and psychosocial parameters.
- Patients treated with the Zephyr Valves experienced significantly more days when their symptoms were “better” and fewer days that were “worse” over 12 months compared to the control group.
- 55% of Zephyr Valve-treated patients achieved a clinically meaningful change in TDI focal score which indicates a return to work/leisure activities previously limited by their breathlessness.1
- By improving patients’ breathlessness, the Zephyr Valves broke the cycle of limited exercise and social activity brought on by the disease. Breaking this cycle is important as reduced physical activity is a predictor of future risk of exacerbation, hospitalization, and early mortality.
“Our analysis confirms what we are seeing at the clinical level; patients treated with the Zephyr Valve are reporting meaningful improvements in their quality of life out to at least one year,” said Mark Dransfield, MD, University of Alabama at Birmingham, and investigator for the LIBERATE Study. “My patients report that they are back to socializing and participating in hobbies like golf and gardening that before the valves had become too difficult because of their disease. I have no other treatment options for COPD patients that can give them this kind of improvement in quality of life without major surgery.”
More than 65 million people suffer with COPD globally, and it is estimated that 3.2 million deaths were caused by the disease in 2015 (5% of all deaths globally). Despite taking the best available medications, many COPD patients, including those with severe emphysema, suffer symptoms of hyperinflation, where air becomes trapped in the lungs, preventing fresh air from entering and thereby causing severe shortness of breath. Patients often have difficulty doing even the simplest tasks like showering or walking up a flight of stairs. The Zephyr Valve, placed through a simple bronchoscopy procedure, reduces this hyperinflation which results in patients being able to breathe more easily and experience less shortness of breath.2
“We are very pleased with this comprehensive report showing that Zephyr Valve treatment not only improves lung function and exercise capacity, but also has a positive and profound impact on patients’ quality of life,” said Glen French, President and Chief Executive Officer. “Our mission as a company is to improve the lives of patients with severe emphysema/COPD and these data clearly shows we are achieving this goal.”
“These data indicate that the Zephyr Valve improves shortness of breath which is the principal symptom limiting exercise in patients with advanced COPD which further leads to activity avoidance,” states Dransfield. “Helping patients breathe easier breaks the downward spiral of symptom-induced inactivity, muscle deconditioning and ensuing weakness, and allows patients to be more active with feelings of well-being, more confidence, and a better quality of life.”
About the Study
This data analysis expands on the data from the LIBERATE Study published in the American Journal of Respiratory and Critical Care Medicine in 2018. LIBERATE included 190 patients with 128 patients randomized to treatment with Zephyr Endobronchial Valves and 62 patients randomized to standard of care (optimized medications and pulmonary rehabilitation). This new analysis focuses on the impact of reduced hyperinflation on many previously unpublished patient-reported outcome measures of breathlessness and symptoms that are most meaningful to patients with COPD as the impact on physical and social activities.
About Bronchoscopic Lung Volume Reduction with the Zephyr Valve
Bronchoscopic lung volume reduction with the Zephyr Valve is a one-time procedure performed through a bronchoscope, which requires no cutting or incisions. During the procedure, an average of four valves are placed in the airways to block off a diseased portion of the lung, which is thereby reduced in size. Reducing hyperinflation and preventing air from getting trapped in the diseased parts of the lung allows the healthier lung tissue to expand and take in more air. This results in patients being able to breathe more easily and experience less shortness of breath. Many patients treated with the Zephyr Valves have reported immediate relief and the ability to go back to doing everyday tasks with greater ease within weeks of treatment.
In its most recent update The Global Initiative for Chronic Obstructive Lung Disease (GOLD), led by experts around the globe upgraded its evidence rating for the use of valves in the treatment of emphysema as level A evidence, the highest rating possible. Both GOLD and UK’s National Institute for Health and Care Excellent (NICE) recommend patients with severe emphysema and hyperinflation be referred for evaluation and consideration for appropriate treatment, which could include either valves or lung volume reduction surgery.
About Pulmonx
Pulmonx Corporation is a commercial-stage medical device company that provides minimally invasive treatment for patients with severe emphysema, a form of COPD. The Pulmonx solution, which is comprised of the Zephyr Endobronchial Valve (Zephyr Valve), the Chartis Pulmonary Assessment System (Chartis System) and the StratX Lung Analysis Platform, is designed to treat severe emphysema patients who, despite medical management, are still profoundly symptomatic and either do not want or are ineligible for surgical approaches. FDA granted the Zephyr Valve a “breakthrough device’ designation, and in June 2018, Pulmonx received FDA pre-market approval to commercialize our Zephyr Valve. The Zephyr Valve is now commercially available in more than 25 countries, with over 76,000 valves used to treat more than 19,000 patients. For more information, visit www.MyLungsMyLife.com
Open access to the full article available at https://www.atsjournals.org/doi/abs/10.1513/AnnalsATS.201909-666OC
________________________________
1 Transitional Dyspnea Index (TDI) measures aspects of daily living as they relate to the amount of breathlessness experienced.
2 Criner G. et al. Am J Respir Crit Care Med. 2018; 198 (9):1151–1164.
View source version on businesswire.com: https://www.businesswire.com/news/home/20200512005517/en/
Source: Pulmonx Corporation