For the roughly 1.5 million Americans per year who survive a traumatic brain injury, health outcomes vary widely. Not only can these injuries lead to a loss of coordination, depression, impulsivity, and difficulty concentrating, but they come with an amplified risk for developing dementia in the future.
Research from Gladstone Institutes reveals that a blood coagulation protein is responsible for initiating toxic inflammation and neuron loss after a major head injury. The findings can inform new treatment strategies for a condition that often presents long-term health challenges.
SAN FRANCISCO, April 19, 2024 /PRNewswire/ -- For the roughly 1.5 million Americans per year who survive a traumatic brain injury, health outcomes vary widely. Not only can these injuries lead to a loss of coordination, depression, impulsivity, and difficulty concentrating, but they come with an amplified risk for developing dementia in the future.
The glaring absence of treatments for such a widespread condition drove a team of scientists at Gladstone Institutes to uncover, on a molecular level, how traumatic brain injuries trigger neurodegeneration—and just as importantly, how to target that process to prevent long-term damage.
"We set out to address the fundamental question of exactly what happens in the brain after injury to ignite the damaging process that destroys neurons," says Jae Kyu Ryu, PhD, a scientific program leader in the lab of Katerina Akassoglou, PhD, at Gladstone Institutes.
Most traumatic brain injuries come as a result of falls, car crashes, or violent assaults, according to the Centers for Disease Control, but many also stem from sports accidents or certain military operations such as explosions. In each case, the external force is strong enough to move the brain within the skull, causing a significant breakdown in the blood-brain barrier and allowing blood to move in.
"We knew that a specific blood protein, fibrin, was present in the brain after traumatic brain injury, but we didn't know until now that it plays a causative role in brain damage after injury," says Ryu, who led the study that appears in the Journal of Neuroinflammation.
Ryu and others in Akassoglou's lab have long investigated how blood that leaks into the brain triggers neurologic diseases, essentially by hijacking the brain's immune system and setting off a cascade of harmful, often-irreversible effects. Fibrin, a protein that normally helps blood coagulate, is the culprit.
"Across many neurological diseases, toxic immune responses in the brain are triggered by blood leaks and drive neurodegeneration," says Akassoglou, a senior investigator at Gladstone and the director of the Center for Neurovascular Brain Immunology at Gladstone and UC San Francisco. "Neutralizing the toxic immune responses in the brain paves the way to new therapies for neurological diseases."
In diseases such as Alzheimer’s and multiple sclerosis, abnormal leaks in the protective blood-brain barrier allow fibrin to seep into areas responsible for cognitive and motor functions causing neurodegeneration. But in this case, the traumatic brain injury itself causes the blood to leak into the brain. The new study showed, for the first time, that fibrin is responsible for turning good immune cells bad, causing dangerous inflammation and unleashing toxins that kill neurons.
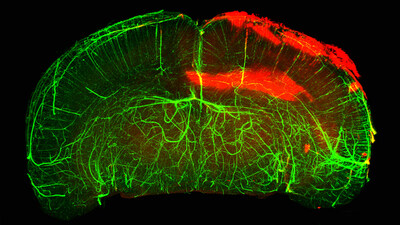
The Gladstone team used state-of-the-art imaging technology to study mouse brains, as well as brains from people who experienced a traumatic brain injury. They also produced three-dimensional imaging of a whole intact mouse brain, showing blood-brain barrier leaks and abundant fibrin in traumatic brain injury. In both mouse and human brains, fibrin was present together with activated immune cells.
"It became clear that fibrin is activating these immune cells," Ryu says. "We realized that we can prevent the toxic effects if we could block fibrin, but we had to do it in a precise way."
The team leveraged genetic tools with a specific mutation in fibrin that can block it from activating immune cells without affecting the protein's beneficial blood-clotting abilities. This is especially critical for traumatic brain injuries, as excessive bleeding into the brain has been known to occur among patients who were taking anticoagulant medications before their injury.
Akassoglou's lab previously developed a drug, a therapeutic monoclonal antibody, that acts only on fibrin's inflammatory properties, without adverse effects on blood coagulation. This fibrin-targeting immunotherapy protects from multiple sclerosis and Alzheimer's disease in mice. A humanized version of this first-in-class fibrin immunotherapy is already in Phase 1 safety clinical trials by Therini Bio.
"It's exciting to have a therapeutic option to neutralize blood toxicity in neurologic diseases," Ryu says. "Future studies are needed to test the effects of the fibrin immunotherapy in traumatic brain injury."
"This study identifies a potential new strategy to diminish the devastating impacts of brain injuries," says Lennart Mucke, MD, director of the Gladstone Institute of Neurological Disease. "Brain injuries can have profound effects on a person's cognitive abilities, emotional health, and motor skills, touching every part of their life. It will be interesting to explore whether blocking the disease-promoting effects of fibrin can improve the outcome of brain surgeries and reduce disability when implemented after traumatic brain injuries have occurred."
About the Study
The study, “Fibrin promotes oxidative stress and neuronal loss in traumatic brain injury via innate immune activation,” appears in the April 15, 2024, issue of Journal of Neuroinflammation. Authors include Terry Dean, Andrew Mendiola, Zhaoqi Yan, Rosa Meza Acevedo, Belinda Cabriga, Katerina Akassoglou, and Jae Kyu Ryu.
The work was supported by the National Institutes of Health, the Thrasher Research Fund Early Career Award, the National Multiple Sclerosis Society, the Kaganov Scholarship for Excellence in Neuroscience, the Conrad N. Hilton Foundation, the Dolby Family, and the Simon Family Trust.
About Gladstone Institutes
Gladstone Institutes is an independent, nonprofit life science research organization that uses visionary science and technology to overcome disease. Established in 1979, it is located in the epicenter of biomedical and technological innovation, in the Mission Bay neighborhood of San Francisco. Gladstone has created a research model that disrupts how science is done, funds big ideas, and attracts the brightest minds.
Media Contact
Kelly Quigley
Gladstone Institutes
Director of Science Communications and Media Relations
kelly.quigley@gladstone.ucsf.edu
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/a-new-therapeutic-target-for-traumatic-brain-injury-302121796.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/a-new-therapeutic-target-for-traumatic-brain-injury-302121796.html
SOURCE Gladstone Institutes