PORTLAND, Ore., May 26, 2016 /PRNewswire-iReach/ -- The Acumed Ankle Plating System 3 and Small Fragment Base Set features a variety of fracture-specific plating options designed to address fractures of the distal tibia and fibula. The system marks the company's continued commitment to provide industry-leading fracture fixation solutions.
The Acumed Ankle Plating System 3 is composed of seven plate families that span the lateral, medial, and posterior malleoli.
Fragment-specific plating of the posterior malleolus is a key area of innovation, as other companies tend to utilize more generic fixation options with less anatomic fit and contour. The importance of fixing the posterior malleolus has been a topic of discussion at nearly every recent orthopaedic conference, with literature demonstrating that the incidence of post-traumatic arthritis increases if the posterior malleolus is involved and that fixation of posterior malleolar fractures provides greater syndesmotic stability.1
One of the system's design surgeons, Anish R. Kadakia, MD, said: "The posterior distal tibia plates uniquely provide fragment-specific fixation for posterior malleolar fractures. The rigid, anatomically contoured plates have allowed me to use them as a reduction tool and, with the low-profile design, have allowed placement adjacent to the PTT and FHL tendons. The plates have changed the way I address posterior malleolar fractures and have helped in decreasing my operative time and achieving articular congruity."
The system includes Hook Plates and Locking Peg Hook Plates, designed to treat challenging avulsion fragments with the option of either hooks or a 2.3 mm cortical peg to capture the fragment distally.
To address disruption of the syndesmosis, the Lateral and Posterolateral Fibula Plates include built-in fixation options, streamlining the technique for the surgeon. The Lateral Fibula Plate includes two screw holes angled 30 degrees anterior to target the center of the tibia and the Posterolateral Fibula Plate includes three scallops that allow for syndesmotic screw heads to sit flush against the plate.
4.0 mm cannulated screws are also included in the Ankle Plating System 3 tray for the treatment of medial malleolar fractures, eliminating the need for an additional tray in the operating room.
The Ankle Plating System 3 is used in combination with the Acumed Small Fragment Base Set. The Small Fragment Base Set includes one-third tubular plates, as well as cut-to-length and bend-to-fit 2.7 mm L-shaped, T-shaped, and straight fragment plates that can also be used to address ankle fractures. A selection of Tension Band Pins and Acutrak® AcuTwist® Compression Screws are also included in the tray.
The 2.7 mm and 3.5 mm variable angle screws included in the Small Fragment Base Set can be angled up to 15 degrees off axis in any direction, helping to accommodate varying patient anatomies, avoid the joint space and other implants, and capture best bone quality.
Together, these systems provide nine plate families, ten screw families, and simplified instrumentation. This provides surgeons with a comprehensive solution to treat nearly any ankle fracture, all within two streamlined trays. The Ankle Plating System 3 and Small Fragment Base Set offers both standard of care, low-cost options in addition to more anatomic, fragment-specific options, allowing the surgeon to select his or her implant based on the severity of the fracture.
For more information about the Ankle Plating System 3, please visit the product page on the Acumed website.
About Acumed
Acumed LLC is a global leader of innovative orthopaedic implant solutions. Founded in 1988, Acumed is headquartered in Hillsboro, Oregon, with offices and a distribution network around the world. Acumed is dedicated to developing products, service methods, and approaches that improve patient care. For more information, please visit www.acumed.net.
Availability
These materials contain information about products that may or may not be available in any particular country or may be available under different trademarks in different countries. The products may be approved or cleared by governmental regulatory organizations for sale or use with different indications or restrictions in different countries. Products may not be approved for use in all countries. Nothing contained on these materials should be construed as a promotion or solicitation for any product or for the use of any product in a particular way which is not authorized under the laws and regulations of the country where the reader is located. Specific questions physicians may have about the availability and use of the products described on these materials should be directed to their particular local sales representative. Specific questions patients may have about the use of the products described in these materials or the appropriateness for their own conditions should be directed to their own physician.
- Liu, G. Posterior Malleolar Fractures: Why Are We Trying to Fix Them All? Presented at: American Orthopaedic Foot & Ankle Society Conference; February 11, 2016; Austin, TX.
To view this video on YouTube, please visit: https://youtu.be/sG2Bb_aXUAo
Media Contact:Lauren Sutton, Acumed, 503.207.1516, lauren.sutton@acumed.net
News distributed by PR Newswire iReach: https://ireach.prnewswire.com
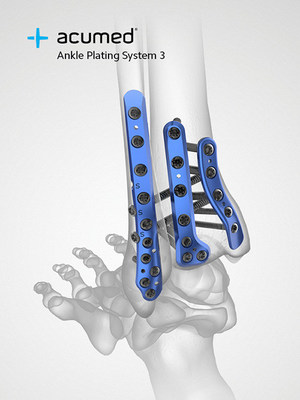
Photo - http://photos.prnewswire.com/prnh/20160525/372397
SOURCE Acumed





