Akston Biosciences Corporation, a developer of new classes of biologic therapeutics, announced that the first of 100 subjects were dosed in an open-label bridging study of AKS-452, its protein subunit COVID-19 vaccine candidate, in India.
- 100 volunteers will participate in AKS-452 study; readouts expected Jan. 2022
- Vaccine is shelf stable for at least six months at room temperature: 25° Celsius (77° Fahrenheit)
- Double-blind placebo-controlled Phase II/III trial to start in Jan. 2022 with 1,500 volunteers
BEVERLY, Mass.--(BUSINESS WIRE)-- Akston Biosciences Corporation, a developer of new classes of biologic therapeutics, announced today that the first of 100 subjects were dosed in an open-label bridging study of AKS-452, its protein subunit COVID-19 vaccine candidate, in India. AKS-452 is shelf stable for at least six months at room temperatures (up to 25° Celsius or 77° Fahrenheit) and maintains its potency for one month at 37° Celsius (99° Fahrenheit).
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20211120005137/en/
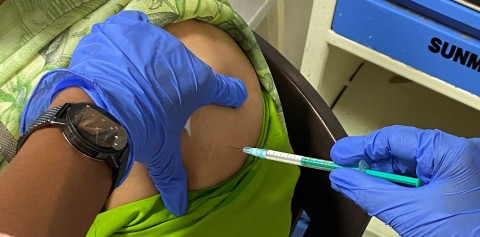
The first volunteer receives Akston Bioscience's COVID-19 vaccine candidate, AKS-452, as part of a Phase II clinical trial in India. The vaccine is shelf-stable for at least six months at room temperatures up to 25°C (77°F). (Photo: Business Wire)
India’s Drug’s Controller General of India (DCGI), Ministry of Health and Family Welfare, approved the open-label bridging study, being conducted by the Supe Heart & Diabetes Hospital and Research Centre, in Nashik, India along with four other sites in the state of Maharashtra. Veeda Clinical Research Ltd., a CRO with experience overseeing complex clinical trials, is managing the study.
The open-label bridging study will be conducted with 100 healthy volunteers, age 18 and older. The first participants were dosed under the supervision of principal investigator, Pravin Dinkar Supe, M.D. founder of the Supe Heart & Diabetes Hospital and Research Centre. A double-blind Phase II/III study will follow with 1,500 healthy volunteers, age 18 and older, across 12 clinical sites in five states across India.
In both studies, healthy volunteers will receive two 90 µg doses 28 days apart. Of the 1,500 participants in the Phase II/III study, 1,150 will receive the two-dose regimen, while the remaining 350 will receive two placebo doses. The first dose will include AKS-452 and an adjuvant, which primes the body’s immune response, with the second dose consisting only of AKS-452.
“The highly promising results from a previous Phase II trial in the Netherlands warrant moving forward with an open-label bridging study and the Phase II/III clinical trial here in India to help speed a low-cost, temperature-resistant vaccine that can be easily manufactured, transported and stored in economically deprived countries in Africa and Asia,” said Dr. Supe. “These countries lack the infrastructure to transport and store the currently-approved vaccines that require ultra-cold conditions.”
“As a second-generation vaccine, AKS-452 has the potential to more easily safeguard the health of populations worldwide against COVID-19. Using our proprietary Fc fusion protein platform, AKS-452 is designed to be well tolerated for primary vaccination and boosting when immunity wanes,” said Todd Zion, Ph.D., President & CEO of Akston Biosciences.
About AKS-452
The AKS-452 is a protein sub-unit vaccine, a type of vaccine used safely and widely for decades. AKS-452 does not contain mRNA technology, viral vectors, or a weakened SARS-CoV-2 virus.
Based on Akston’s proprietary Fc fusion protein platform, AKS-452 is a SARS-CoV-2 vaccine candidate designed to induce a Th1/Th2 mixed immune response in patients against the Receptor Binding Domain (RBD) of the novel coronavirus spike protein. Being the primary locus for infection, the RBD is highly conserved among mutated forms of the virus, and non-clinical testing has demonstrated robust antibody neutralization of SARS-CoV-2 variants. The vaccine has been engineered to use standard, low-cost, antibody manufacturing techniques, such that a single production line could be capable of producing over one billion doses per year.
About Akston Biosciences
Akston Biosciences Corporation leverages its novel fusion protein platform to develop and manufacture new classes of biologics, including vaccines, ultra-long-acting insulins, and autoimmune disease therapies. Founded by the team that developed the world’s first clinical glucose-responsive insulin at SmartCells, Inc. (sold to Merck & Co.), Akston has partnered with Dechra Pharmaceuticals PLC (DPH) to commercialize once-a-week canine and feline insulin therapies. It operates a GMP biologics manufacturing facility and research laboratory at its Beverly, Mass. location. www.akstonbio.com.
About Veeda Clinical Research Limited
Veeda Clinical Research Limited (“Veeda”) is one of the largest independent, full-service clinical research organizations, by revenue in India, headquartered in Ahmedabad, India. Veeda offers a range of bioequivalence studies and early and late phase clinical trials. Veeda has successfully completed several regulatory inspections and is approved by USFDA, UK MHRA, ANVISA (Brazil), and WHO. Veeda has experience in conducting complex clinical studies. www.veedacr.com.
View source version on businesswire.com: https://www.businesswire.com/news/home/20211120005137/en/
Linda Pendergast-Savage
Birnbach Communications for Akston Biosciences
+1-508-224-7905
lpendergastsavage@birnbachcom.com
Source: Akston Biosciences Corporation
The first volunteer receives Akston Bioscience's COVID-19 vaccine candidate, AKS-452, as part of a Phase II clinical trial in India. The vaccine is shelf-stable for at least six months at room temperatures up to 25°C (77°F). (Photo: Business Wire)
View this news release and multimedia online at:
http://www.businesswire.com/news/home/20211120005137/en