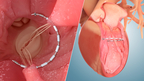
Ancora Heart, Inc. and egnite, Inc. today announced a strategic partnership to accelerate clinical trial enrollment for Ancora Heart’s CORCINCH-HF pivotal clinical trial, which is evaluating the safety and efficacy of the AccuCinch® Ventricular Restoration System.
Use of artificial intelligence technology efficiently identifies eligible patient candidates for pivotal clinical trial; program launched at The Christ Health Network
SANTA CLARA, Calif. & ALISO VIEJO, Calif.--(BUSINESS WIRE)-- Ancora Heart, Inc., a medical device company developing a novel transcatheter device-based therapy to address heart failure (HF), and egnite, Inc., a leading digital health company in the field of cardiovascular care, today announced a strategic partnership to accelerate clinical trial enrollment for Ancora Heart’s CORCINCH-HF pivotal clinical trial, which is evaluating the safety and efficacy of the AccuCinch® Ventricular Restoration System. The partnership brings together the cutting-edge technology and expertise of both organizations with the goal of delivering innovative therapies to patients faster. The Christ Health Network in Cincinnati, OH is the first site in Ancora Heart’s CORCINCH-HF trial to utilize egnite’s Trial Accelerator artificial intelligence (AI) technology to help identify eligible patients.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20230419005428/en/
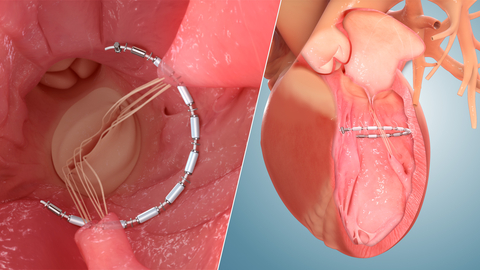
Ancora Heart’s AccuCinch® Ventricular Restoration System device is designed to restore the structure and function of the enlarged left ventricle of the heart, thereby addressing the fundamental issue in the progression of heart failure in patients with reduced ejection fraction (HFrEF). The AccuCinch System is an investigational device, which is currently being studied in the CORCINCH-HF pivotal trial, a prospective, randomized, open-label, multicenter, international, clinical safety and efficacy investigation. (Graphic: Business Wire)
“Our goal with the development of the AccuCinch System is to provide a minimally invasive treatment option to further improve the lives of heart failure patients whose symptoms progress despite guideline directed medical therapy,” said Mark Miles, chief commercial officer of Ancora Heart. “This partnership with egnite and The Christ Health Network will allow us to deploy AI tools such as egnite’s Trial Accelerator and expedite identification of eligible candidates for the CORCINCH-HF trial.”
egnite’s Trial Accelerator technology will also be leveraged at other participating CORCINCH-HF sites to drive patient identification and enrollment for the pivotal trial. The innovative solution can accelerate clinical trial enrollment through AI and big data processing, automatically screening hundreds of thousands of patients against trial parameters to help identify more trial candidates without hospital staff dedicating hours to manual chart review.
“Identifying patient candidates for clinical trial participation can be a complex task that requires many hours to review medical charts in the search for matching clinical criteria,” said Dr. Dean Kereiakes, president of The Christ Hospital Heart and Vascular Institute and co-principal investigator for CORCINCH-HF. “The use of AI technology streamlines the trial screening process by identifying candidates more quickly and efficiently, thereby freeing up critical resources needed for clinical trial success.”
“We are thrilled to partner with leading life sciences organizations like Ancora Heart and apply our novel technology to accelerate clinical trial enrollment,” said Joel Portice, president and chief executive officer of egnite. “We believe this collaboration is a significant step forward for patients and are excited to be at the forefront of cardiovascular care.”
About Ancora Heart, Inc.
Ancora Heart is a medical device company dedicated to providing new treatment options for people with heart failure (HF). The company’s proprietary AccuCinch® Ventricular Restoration System is the only completely transcatheter device designed to restore the structure and function of the enlarged left ventricle of the heart, thereby addressing the fundamental issue in the progression of heart failure in patients with reduced ejection fraction (HFrEF). The AccuCinch System is an investigational device, which is currently being studied in the CORCINCH-HF pivotal trial, a prospective, randomized, open-label, multicenter, international, clinical safety and efficacy investigation (ClinicalTrials.gov Identifier: NCT04331769). Ancora Heart is a privately held company located in Santa Clara, Calif. For more information, please visit www.ancoraheart.com.
About egnite, Inc.
egnite is a visionary digital health company committed to advancing the health of our society through innovative cardiovascular solutions. egnite uses AI-driven algorithms and big data to produce business intelligence for healthcare, elevating the role of data in critical decisions. The company partners with leading hospitals and life sciences providers to transform care delivery for cardiovascular patients. The company is based in Aliso Viejo, Calif., for more information, visit www.egnitehealth.com.
About The Christ Hospital Health Network
The Christ Hospital Health Network consists of an acute care hospital located in Mt. Auburn, a remote hospital location in Liberty Township, five ambulatory outpatient centers and dozens of medical offices throughout the region. For more than 130 years, The Christ Hospital has provided compassionate care to those it serves. Made up for more than 1,300 physicians and more than 6,500 team members, our mission is to improve the health of our community by providing exceptional outcomes in an affordable way. The Network was recognized by U.S. News & World Report as the #1 hospital in the Cincinnati Region, named to Newsweek’s World’s Best Hospitals list in 2023. It is also a Press Ganey Guardian of Excellence Award recipient, which recognizes top-performing healthcare organizations that achieve the 95th percentile or above of performance in patient experience.
egnite, egnite, Inc., and the spark logo are trademarks of egnite, Inc. All other trademarks are the property of their respective owners.
View source version on businesswire.com: https://www.businesswire.com/news/home/20230419005428/en/
Contacts
egnite
Rachel Stein
949-309-1325
rachel@healthandcommerce.com
Ancora Heart
Jessica Volchok
310-849-7985
jessica@merrymancommunications.com
The Christ Health Network
James Buechele
513- 263-1443
James.Buechele@thechristhospital.com
Source: Ancora Heart, Inc.
Smart Multimedia Gallery
Ancora Heart’s AccuCinch® Ventricular Restoration System device is designed to restore the structure and function of the enlarged left ventricle of the heart, thereby addressing the fundamental issue in the progression of heart failure in patients with reduced ejection fraction (HFrEF). The AccuCinch System is an investigational device, which is currently being studied in the CORCINCH-HF pivotal trial, a prospective, randomized, open-label, multicenter, international, clinical safety and efficacy investigation. (Graphic: Business Wire)
egnite Inc.'s Trial Accelerator technology is helping Ancora Heart drive patient identification and enrollment for its CORCINCH-HF pivotal trial, which is evaluating the use of the AccuCinch® Ventricular Restoration System in patients with heart failure. Using artificial intelligence and big data processing, the technology screens hundreds of thousands of patients against trial parameters to help identify more trial candidates without hospital staff dedicating hours to manual chart review. (Graphic: Business Wire)