New data provides evidence of adaptive cellular (CD8+ cells) and balanced (Th1/Th2) immune responses STARR™ mRNA elicits anti-spike protein antibodies (IgG), higher than conventional mRNA at all doses SAN DIEGO, May 08, 2020 (GLOBE NEWSWIRE) -- Arcturus Therapeutics Holdings Inc. (the “Company”, “Arcturus”, Nasdaq: ARCT), a leading clinical-stage messenger RNA medicines company focused on the discovery, development and commercialization of therapeutics for rare diseases and vaccines, tod
New data provides evidence of adaptive cellular (CD8+ cells) and balanced (Th1/Th2) immune responses
STARR™ mRNA elicits anti-spike protein antibodies (IgG), higher than conventional mRNA at all doses
SAN DIEGO, May 08, 2020 (GLOBE NEWSWIRE) -- Arcturus Therapeutics, Inc.. (the “Company”, “Arcturus”, Nasdaq: ARCT), a leading clinical-stage messenger RNA medicines company focused on the discovery, development and commercialization of therapeutics for rare diseases and vaccines, today announced new supportive preclinical data, providing evidence for an adaptive cellular (CD8+ cells) and balanced (Th1/Th2) immune response data from the Company’s COVID-19 vaccine program (LUNAR-COV19). These new results augment previously disclosed preclinical data demonstrating a strong antibody response (anti-spike protein IgG and 100% virus neutralization at a very low vaccine dose) from the program. Together, the available data indicate that LUNAR-COV19 is effectively activating the two fundamentally important components of the adaptive immune response, providing strong support for human vaccine clinical trials, which are on track to begin this summer.
These scientific data supporting robust immunogenicity of LUNAR-COV19 were measured by investigators at the Duke-NUS Medical School in Singapore.
“These extended preclinical studies, conducted in partnership with Arcturus, establish a comprehensive and highly compelling package of data to support clinical trials,” said Professor Ooi Eng Eong, Deputy Director of the Emerging Infectious Diseases Programme at Duke-NUS Medical School. “The new preclinical data for LUNAR-COV19 vaccine are very promising and represents an even more compelling case for clinical trials.”
Pad Chivukula, Ph.D., Chief Scientific Officer of Arcturus Therapeutics, stated “A vaccine approach that elicits a broader immune response by activating both humoral (i.e., antibodies) and cellular (T-cells) immunity has the potential to provide more potent clinical protection. Our preclinical LUNAR-COV19 data confirms this broad immune response.”
Joseph Payne, President & CEO of Arcturus added, “Self-replicating mRNA significantly increases spike protein expression as compared to conventional mRNA, yielding many-fold higher seroconversion rates. The favorable results seen, even at the very low 0.2 µg dose, gives us increased conviction that our vaccine candidate may be seroprotective at the lower doses that we plan to investigate in the clinic.”
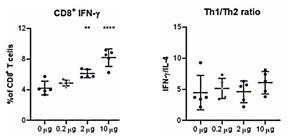
An additional study evaluating cell-mediated immunogenicity has been recently completed at Professor Ooi Eng Eong’s lab at Duke-NUS. The results showed a dose dependent CD8+ T-cell response, with a clear response observed at all doses, as well as a balanced Th1/Th2 CD4+ T-cell response (intracellular cytokine (IFN-γ/IL-4) staining). The percent of CD8+ T-cells increased from the 4% baseline to 8% with increasing doses of STARR™ mRNA. The Th1/Th2 ratio for T-helper cells (CD4+) shows a strong TH1 response which does not change with increasing dose, indicating that the immune response remains balanced across all dose levels.
STARR™ mRNA (LUNAR-COV19) Induces Adaptive Cellular Immunity
| Single Dose (µg) |
LUNAR-COV19 (STARR™ mRNA) After 7 Days | |
| % CD8+ T-Cells | Th1/Th2 | |
| 0 | 4.0 | 4.6 |
| 0.2 | 4.5 | 5.3 |
| 2 | 6.0 | 5.0 |
| 10 | 8.0 | 6.0 |
A single administration of LUNAR-COV19 STARR™ mRNA induced a higher anti-spike protein IgG response than conventional mRNA at equivalent doses, and particularly at lower doses. The IgG response also continued to increase at a much greater rate over the 30-day post vaccination period than conventional mRNA.
Immunogenicity1 (Anti-spike protein IgG) – STARR™ mRNA vs. Conventional
| Single Dose (µg) |
LUNAR® Delivery | |||||
| STARR™ mRNA (MFI)2 | Conventional mRNA (MFI)2 | |||||
| Day 10 | Day 19 | Day 30 | Day 10 | Day 19 | Day 30 | |
| 0.2 | 361 | 850 | 1887 | 110 | 154 | 260 |
| 2 | 1217 | 1885 | 3062 | 367 | 267 | 327 |
| 10 | 1413 | 2895 | 4789 | 1077 | 1053 | 1456 |
1Immunogenicity is defined as the induction of multiple antibodies (IgG) that bind to the spike protein following intramuscular administration.
2 Mean Fluorescence Intensity (MFI); Serum diluted 1 to 2000.
A vaccine approach that elicits a broad and balanced immune response by activating both humoral and cellular immunity has the potential to provide more effective protection. An important advantage of our self-replicating mRNA vaccine is the potential to activate both of these important components of adaptive immunity and this preclinical LUNAR-COV19 data confirms a robust and balanced immune response.
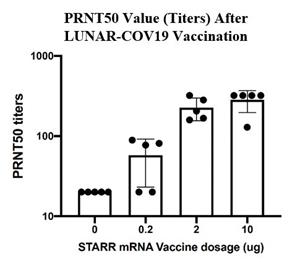
Seroconversion was determined using a quantitative plaque reduction neutralization assay (using SARS-CoV-2 Singapore Clinical Isolate), which measured neutralizing antibody titers are detected in serum at day 30. Rodents were immunized with a single intramuscular dose (0.2, 2, and 10 µg) of LUNAR-COV19 vaccine.
At day 30 post vaccination, 80% of mice vaccinated with 0.2 µg LUNAR-COV19 elicited antibodies that neutralized 50% of SARS-CoV-2 at titers 20 and above. The geometric mean titer of the 4 animals with titers >20 is 57.72 (SD = 2.032). At the same time point, 100% animals vaccinated with 2 µg of LUNAR-COV19 developed antibody titers >20, with geometric mean titer of 217.9 (SD = 1.365). The 10 µg dose of LUNAR-COV19 vaccine produced titers of 320 or greater in 80% of animals, which was the upper limit of dilution of this test. The concentration of serum to reduce the number of plaques by 50% compared to the serum free virus gives the measure of how much antibody is present or how effective it is. This measurement is denoted as the PRNT50 value. The plaque reduction neutralization test is used to quantify the titer of neutralizing antibody for a virus.
About Arcturus Therapeutics
Founded in 2013 and based in San Diego, California, Arcturus Therapeutics Holdings Inc. (Nasdaq: ARCT) is a clinical-stage mRNA medicines and vaccines company with enabling technologies: (i) LUNAR® lipid-mediated delivery, (ii) STARR™ mRNA Technology and (iii) mRNA drug substance along with drug product manufacturing expertise. Arcturus’ diverse pipeline of RNA therapeutic candidates includes programs to potentially treat Ornithine Transcarbamylase (OTC) Deficiency, Cystic Fibrosis, Glycogen Storage Disease Type 3, Hepatitis B, non-alcoholic steatohepatitis (NASH) and a self-replicating mRNA vaccine for SARS-CoV-2. Arcturus’ versatile RNA therapeutics platforms can be applied toward multiple types of nucleic acid medicines including messenger RNA, small interfering RNA, replicon RNA, antisense RNA, microRNA, DNA, and gene editing therapeutics. Arcturus’ technologies are covered by its extensive patent portfolio (187 patents and patent applications, issued in the U.S., Europe, Japan, China and other countries). Arcturus’ commitment to the development of novel RNA therapeutics has led to collaborations with Janssen Pharmaceuticals, Inc., part of the Janssen Pharmaceutical Companies of Johnson & Johnson, Ultragenyx Pharmaceutical, Inc., Takeda Pharmaceutical Company Limited, CureVac AG, Synthetic Genomics Inc., Duke-NUS, Catalent Inc., and the Cystic Fibrosis Foundation. For more information visit www.ArcturusRx.com
About STARR™ Technology
The STARR™ Technology platform combines self-replicating mRNA with LUNAR®, a leading nanoparticle delivery system, into a single solution to produce proteins inside the human body. The versatility of the STARR™ Technology affords its ability upon delivery into the cell to generate a protective immune response or drive therapeutic protein expression to potentially prevent against or treat a variety of diseases. The self-replicating RNA-based therapeutic vaccine triggers rapid and prolonged antigen expression within host cells resulting in protective immunity against infectious pathogens. This combination of the LUNAR® and STARR™ technology is expected to provide lower dose requirements due to superior immune response, sustained protein expression compared to non-self-replicating mRNA-based vaccines and potentially enable us to produce vaccines more quickly and simply.
Forward Looking Statements
This press release contains forward-looking statements that involve substantial risks and uncertainties for purposes of the safe harbor provided by the Private Securities Litigation Reform Act of 1995. Any statements, other than statements of historical fact included in this press release, including those regarding the Company’s expected performance, the Company’s development of any specific novel mRNA therapeutics, the Company’s efforts to develop a vaccine against COVID-19 based on the Company’s mRNA therapeutics, the forecasted safety, efficacy or reliability of a vaccine against COVID-19,were one to be successfully developed based on the Company’s mRNA therapeutics, the timing and availability of a vaccine against COVID-19 were one to be successfully developed based on the Company’s mRNA therapeutics, the potential initiation of human trials of a vaccine against COVID-19 based on the Company’s mRNA therapeutics, the timing of initiation of human trials of a vaccine against COVID-19 based on the Company’s mRNA therapeutics, the potential market impact of a vaccine against COVID-19 based on the Company’s mRNA therapeutics and the impact of general business and economic conditions are forward-looking statements. Arcturus may not actually achieve the plans, carry out the intentions or meet the expectations or projections disclosed in any forward-looking statements such as the foregoing and you should not place undue reliance on such forward-looking statements. Such statements are based on management’s current expectations and involve risks and uncertainties, including those discussed under the heading "Risk Factors" in Arcturus’ Annual Report on Form 10-K for the fiscal year ended December 31, 2019, filed with the SEC on March 16, 2020 and in subsequent filings with, or submissions to, the SEC. No assurances can be given that any results reported in pre-clinical studies can be replicated in further studies or in human beings, or that a vaccine can or will ever be developed or approved using the Company’s technology. Except as otherwise required by law, Arcturus disclaims any intention or obligation to update or revise any forward-looking statements, which speak only as of the date they were made, whether as a result of new information, future events or circumstances or otherwise.
Contact
Arcturus Therapeutics
Neda Safarzadeh
(858) 900-2682
IR@ArcturusRx.com
Kendall Investor Relations
Carlo Tanzi, Ph.D.
(617) 914-0008
ctanzi@kendallir.com
Photos accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/06a2e8fe-5e76-47e0-897e-7303baeacf62
https://www.globenewswire.com/NewsRoom/AttachmentNg/3879704c-a063-4570-aa96-2e44a1341a6d