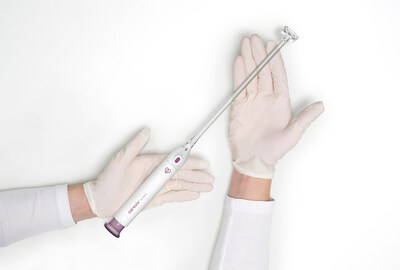
ASPIVIX SA today announced that Carevix™, its novel Cervical Stabilizer, has received 510(k) clearance from the U.S. Food and Drug Administration (FDA).
|
LAUSANNE, Switzerland, Feb. 2, 2023 /PRNewswire/ -- ASPIVIX SA, an innovator and developer of medical technologies to advance gynecological care, today announced that Carevix™, its novel Cervical Stabilizer, has received 510(k) clearance from the U.S. Food and Drug Administration (FDA). This next generation device for routine procedures in gynecology will allow millions of women across the USA access to significantly less painful treatments and IUD insertions Carevix™ is an atraumatic cervical stabilizer that utilizes a gentle approach to reduce pain and bleeding in multiple transcervical procedures, such as, Intrauterine Device insertions. In our ADVANCE Women, single-blinded, randomized, multicentric, comparative study of 100 women who underwent an IUD insertion with either the Carevix™ device or a traditional cervical tenaculum, women reported statistically significant results with up to 73% reduction of pain scores and 78% reduction of bleeding occurrences in favor of the Carevix™ device.1 Carevix™ is the result of our constant commitment to make gynecology, now modern. Mathieu Horras, Chief Executive Officer of ASPIVIX emphasized: "With the 510(k) clearance of Carevix™, a design-award winning device, we will provide our U.S. customers with an innovative and easy-to-use system that brings a gentler alternative to a century-old gynecological tool. Extensive research was incorporated into the development of Carevix™ so we know the unique and differentiating features it demonstrates with significant less pain and bleeding that has the potential to dramatically improve the IUD adoption and placement experience for millions of American women." About CarevixTM About ASPIVIX Visit www.aspivix.com or stay informed www.aspivix.com/stay-informed/ Media Contact: References Photo - https://mma.prnewswire.com/media/1993207/Carevix.jpg
SOURCE Aspivix SA |