Revelle Aesthetics, Inc. (Revelle) – a VC-backed Silicon Valley MedTech company – achieves an extended FDA clearance for its precision cellulite release device, Avéli™.
| --Avéli Offers Women Meaningful and Lasting Results for Cellulite, One of the Most Bothersome Lower Body Contouring Challenges-- MOUNTAIN VIEW, Calif., Sept. 1, 2022 /PRNewswire/ -- Revelle Aesthetics, Inc. (Revelle) – a VC-backed Silicon Valley MedTech company – achieves an extended FDA clearance for its precision cellulite release device, Avéli™. Marking another key milestone for the Company, Avéli is now indicated for long-term reduction in the appearance of cellulite in the buttocks and thigh areas of adult females as supported by clinical data demonstrating treatment benefits through one year of observation. Experience the full interactive Multichannel News Release here: “For far too long, millions of women have struggled to feel comfortable in their own skin because of their cellulite and have been settling for less than effective treatment options. Avéli is setting out to change this by finally providing women with a meaningful and lasting solution that targets their cellulite in the areas that have frustrated them the most, their buttocks and thighs,” said Caroline Van Hove, President and CEO of Revelle Aesthetics. “Equally as exciting is the role that we believe Avéli will play as a cornerstone in the latest and fastest growing category in medical aesthetics, lower body rejuvenation.” Cellulite is a depression of any shape or size with a defined edge, typically on the buttocks or thighs, and affects up to 90% of women. A major underlying cause of cellulite is the complex structure of fibrous septa bands that tether the skin. Some septa stiffen or shrink over time and lead to the dimples we see on the skin’s surface. Unlike many current cellulite options, Avéli addresses cellulite from the inside-out. It is the only minimally invasive procedure that allows a provider to identify which of the fibrous septa bands are causing a cellulite dimple, and then confirm in real-time they are releasing those targeted septa to deliver visibly smoother skin. Results are visible quickly after a single in-office procedure with little-to-no downtime. “Recent data from The Aesthetics Society confirms what we are seeing in our practices every day. Women’s aesthetic concerns have quickly descended below the face and chest with body contouring procedures being the fastest growing segment of our business,” shares Laurie Casas, MD, FACS, board-certified plastic surgeon, founder of Casas Aesthetic Plastic Surgery in Chicago, IL, and Clinical Associate, Section of Plastic and Reconstructive Surgery The University of Chicago Medicine. “The relationship women have with their body is extremely personal and emotional. Unwanted fat, skin laxity and cellulite are the most common complaints I hear. That is why effective treatments like Avéli have such a life-changing impact.” Avéli is now available nationwide, growing rapidly in plastic surgery and dermatology practices that are focused on the rising needs in body contouring. Revelle’s approach to successful integration of Avéli into practices consists of robust clinical proctoring along with dedicated high-touch marketing and educational support. This focus has resulted in hundreds of compelling real-world outcomes, and robust scientific presentations among key industry congresses and publications in top-tier journals. “There is a real need out there with women; we as physicians just need to start having the cellulite conversation with them,” says Dr. Nazanin Saedi, a board-certified dermatologist at Dermatology Associates of Plymouth Meeting in Philadelphia, PA, and member of the Board of Directors for the American Society for Dermatologic Surgery. “Before Avéli, I didn’t feel I had much to offer them that was impressive and reliable. Avéli is a very straightforward and lasting solution to something we have struggled to treat for so long. When I tell my patients how Avéli works, it is an easy conversation – it just makes sense to them. Results in a one-time treatment and the lack of capital equipment make Avéli an economically attractive, easy-to-integrate option for us and our patients.” Visit MyAveli.com or Instagram @aveli to learn more about Avéli and join the cellulite conversation. ABOUT REVELLE AESTHETICS Revelle Aesthetics is a Silicon Valley-based MedTech company focused on innovating smart solutions that address the root causes of women’s most bothersome aesthetic concerns. The company is committed to developing precision technologies that deliver meaningful results for women and reliable outcomes for physicians. Revelle Aesthetics’ first device, Avéli™, is FDA-cleared for long-term reduction in the appearance of cellulite in the buttocks and thigh areas of adult females as supported by clinical data demonstrating treatment benefits through one year of observation. Refer to the Instructions for Use at myaveli.com/IFU for safety information and clinical performance data summary. Founded in 2018 and based in Mountain View, California, Revelle Aesthetics is a privately held company backed by top-tier venture capital firms New Enterprise Associates and KCK Medtech. For more information visit www.RevelleAesthetics.com. Media Contact: 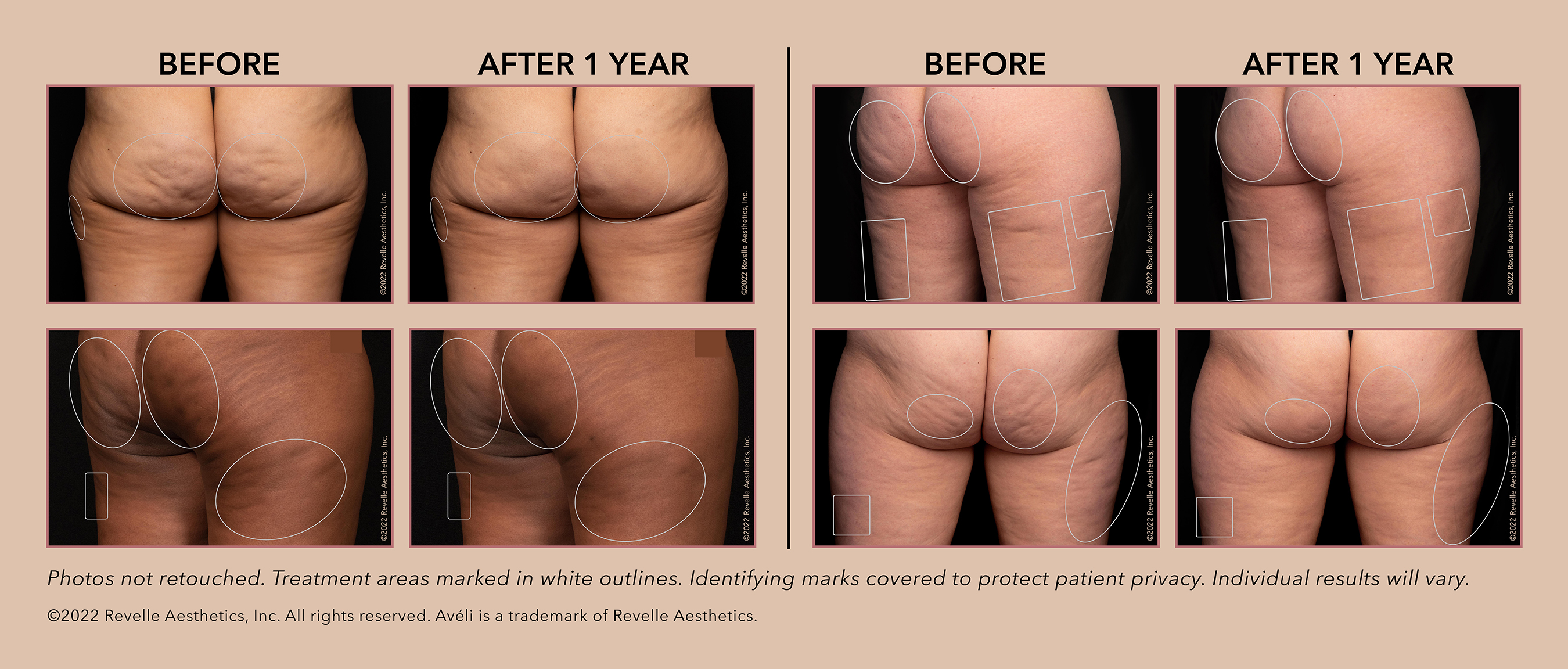
SOURCE Revelle Aesthetics |




