Biohaven Therapeutics Ltd., a subsidiary of Biohaven Pharmaceutical Holding Company Ltd., along with CD3 and LICR at KU Leuven, announced that they have entered into an exclusive global license and research agreement to develop and commercialize first-in-class TRPM3 antagonists to address the growing proportion of people worldwide living with chronic pain disorders.
- Biohaven acquires exclusive global rights to develop and commercialize first-in-class transient receptor potential melastatin-3 (TRPM3) channel antagonists
- The lead candidate, BHV-2100, is planned to enter clinical development in the first half of 2023 for the treatment of neuropathic pain
NEW HAVEN, Conn. and LEUVEN, Belgium, Jan. 10, 2022 /PRNewswire/ -- Biohaven Therapeutics Ltd., a subsidiary of Biohaven Pharmaceutical Holding Company Ltd. (NYSE: BHVN), along with CD3 and LICR at KU Leuven, announced today that they have entered into an exclusive global license and research agreement to develop and commercialize first-in-class TRPM3 antagonists to address the growing proportion of people worldwide living with chronic pain disorders. The TRPM3 antagonist platform was discovered at the Centre for Drug Design and Discovery (CD3) and the Laboratory of Ion Channel Research (LICR) at KU Leuven.
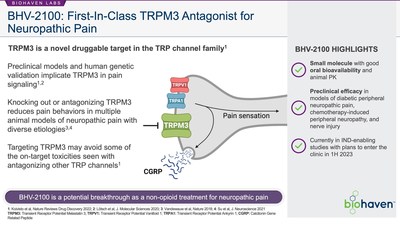
BHV-2100 is the lead TRPM3 antagonist from the platform and an orally-bioavailable small molecule TRPM3 antagonist. TRPM3 is a cation channel involved in pain signaling and a novel target for the treatment of pain discovered at KU Leuven’s LICR. Consistent with the role of TRPM3 in pain, data generated at KU Leuven demonstrated that BHV-2100 may reduce pain behaviors in animal models without the detrimental side-effects seen with other pain management approaches and mechanisms.
Vlad Coric, M.D., Chief Executive Officer and Chairman of the Board of Biohaven Pharmaceutical Holding Company Ltd., said, “We are pleased to expand our pipeline of novel compounds targeting pain disorders, which already includes our migraine focused CGRP franchise, through this collaboration with CD3 and KU Leuven. There is an urgent unmet need for safe, effective treatments for pain, and TRPM3 antagonism is a promising approach to advance new pain therapies. Based on the extensive characterization of the mechanism and encouraging preclinical data generated to date by KU Leuven, we are excited to collaborate with these academic leaders to bring BHV-2100 into clinical development.”
Thomas Voets, professor at KU Leuven and group leader at the VIB-KU Leuven Center for Brain & Disease Research, said “We are excited to collaborate with Biohaven and look forward to advancing our TRPM3 program towards clinical applications for the benefit of chronic pain sufferers.” Joris Vriens, professor at KU Leuven added: “Our fundamental research has shown that TRPM3 is a promising target for a novel type of analgesics, and this partnership will give us the necessary resources to advance our TRPM3-targeting compounds to the clinical stage.”
Charlie Conway, PhD, Chief Scientific Officer of Biohaven Pharmaceuticals, Inc. remarked, “Professors Voets and Vriens at KU Leuven have been pioneers in elucidating and validating the pivotal role of TRPM3 in pain signaling. Their research provides a strong rationale for advancing TRPM3 modulators to treat pathological pain. I’m excited to carry BHV-2100 forward as the first candidate in a potentially transformative class of pain therapies.”
Under the agreement, Biohaven Therapeutics receives exclusive global rights to develop, manufacture and commercialize KU Leuven’s small-molecule TRPM3 antagonists. The portfolio includes the lead candidate, henceforth known as BHV-2100, which has demonstrated promising efficacy in preclinical pain models and will be the first to advance towards Phase 1 studies. Biohaven Therapeutics will support further basic and translational research at KU Leuven on the role of TRPM3 in pain and other disorders. KU Leuven will receive an upfront payment and is eligible to receive additional development, regulatory, and commercialization milestones. In addition, KU Leuven will be eligible to receive mid-single digit royalties on net sales of products resulting from the collaboration.
Patrick Chaltin, Managing Director CD3 added: “We are extremely pleased that our long lasting and successful partnership with LICR has enabled the TRPM3 antagonist program to achieve this important milestone. Biohaven has a proven track record of successfully developing and launching novel therapies, and we’re excited to work with Biohaven through this global license and research collaboration agreement.”
About Biohaven
Biohaven Pharmaceutical Holding Company Ltd., parent company of Biohaven Therapeutics, is a commercial-stage biopharmaceutical company with a portfolio of innovative, best-in-class therapies to improve the lives of patients with debilitating neurological and neuropsychiatric diseases, including rare disorders. Biohaven’s Neuroinnovation™ portfolio includes FDA-approved NURTEC® ODT (rimegepant) for the acute and preventive treatment of migraine and a broad pipeline of late-stage product candidates across three distinct mechanistic platforms: CGRP receptor antagonism for the acute and preventive treatment of migraine; glutamate modulation for obsessive-compulsive disorder, Alzheimer’s disease, and spinocerebellar ataxia; and myeloperoxidase (MPO) inhibition for amyotrophic lateral sclerosis. More information about Biohaven is available at www.biohavenpharma.com.
About KU Leuven, CD3 and LICR
As the number one European university for innovation, KU Leuven actively invests in launching innovative technologies in the commercial market by creating spin-off companies, securing and licensing intellectual property, and collaborating with industry. KU Leuven supports researchers and students in transforming their innovative ideas and technologies into commercial products and services that impact people’s lives across the globe. KU Leuven Research & Development (LRD) is KU Leuven’s technology transfer office. Ever since 1972, LRD has been building bridges between science and industry. For more information, please visit: lrd.kuleuven.be/en
The Centre for Drug Design and Discovery (CD3) translates innovative science into promising drug discovery programs that are well qualified for further development by pharmaceutical or biotech companies. CD3 brings expert drug discovery capabilities and financial means to academic research groups and small companies in order to discover innovative drugs. Supported by KU Leuven Research & Development and the European Investment Fund, CD3 has entered into several successful partnerships with pharmaceutical companies and also integrated drug discovery programs in spin-off companies. For more information, please visit www.cd3.be.
The Laboratory of Ion Channel Research (LICR) studies ion channels in all their facets, from their molecular and cellular mode of action to their role in health and disease. The central focus is on Transient Receptor Potential (TRP) channels, a superfamily of cation channels involved in a wide array of physiological processes, including sensory processing and pain signaling. Through intense collaboration with clinical researchers and medicinal chemists, LICR aims at translating fundamental insights on TRP channel biology towards new therapies. For more information, please visit gbiomed.kuleuven.be/LICR and voetslab.sites.vib.be.
Forward-looking Statement
This news release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. The use of certain words, including “believe”, “may” and “will” and similar expressions, are intended to identify forward-looking statements. These forward-looking statements involve substantial risks and uncertainties, including statements that are based on the current expectations and assumptions of Biohaven’s management about BHV-2100 and TRPM3 as a novel target for the treatment of pain. Biohaven may not actually achieve the plans, intentions or expectations disclosed in the forward-looking statements and you should not place undue reliance on Biohaven’s forward-looking statements. Various important factors could cause actual results or events to differ materially from those that may be expressed or implied by our forward-looking statements. Additional important factors to be considered in connection with forward-looking statements are described in the “Risk Factors” section of the Company’s Annual Report on Form 10-K filed with the Securities and Exchange Commission on March 1, 2021, and the Company’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on November 9, 2021. The forward-looking statements are made as of this date and Biohaven does not undertake any obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
NURTEC and NURTEC ODT are registered trademarks of Biohaven Pharmaceutical Ireland DAC.
Neuroinnovation is a trademark of Biohaven Pharmaceutical Holding Company Ltd.
Biohaven Contact:
Dr. Vlad Coric
Chief Executive Officer
Vlad.Coric@biohavenpharma.com
Media Contact:
Mike Beyer
Sam Brown Inc.
mikebeyer@sambrown.com
312-961-2502
KU Leuven Contact:
Patrick Chaltin
Managing Director CD3
patrick.chaltin@kuleuven.be
+32 16 852 605
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/biohaven-enters-exclusive-license-and-research-collaboration-agreement-with-ku-leuven-to-advance-first-in-class-trpm3-antagonists-for-the-treatment-of-pain-301456862.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/biohaven-enters-exclusive-license-and-research-collaboration-agreement-with-ku-leuven-to-advance-first-in-class-trpm3-antagonists-for-the-treatment-of-pain-301456862.html
SOURCE Biohaven Pharmaceutical Holding Company Ltd.
Company Codes: NYSE:BHVN