Biohaven today announced positive topline results from the second pivotal clinical trial (NCT04571060) evaluating the safety and efficacy of its investigational therapy, intranasal zavegepant, for the acute treatment of migraine in adults.
- Intranasal zavegepant 10 mg met the study’s co-primary endpoints and demonstrated statistically significant superiority versus placebo on a total of 15 consecutive, prespecified primary and secondary outcome measures in the acute treatment of migraine
|
| [06-December-2021] |
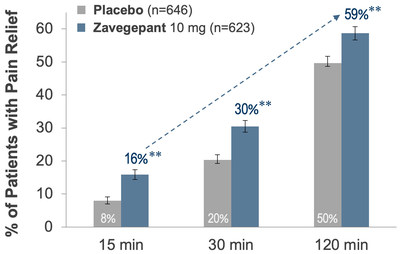
| NEW HAVEN, Conn., Dec. 6, 2021 /PRNewswire/ --Biohaven Pharma Holding Company Ltd. (NYSE: BHVN) today announced positive topline results from the second pivotal clinical trial (NCT04571060) evaluating the safety and efficacy of its investigational therapy, intranasal zavegepant, for the acute treatment of migraine in adults. The Phase 3 study achieved its co-primary regulatory endpoints of pain freedom and freedom from most bothersome symptom at 2 hours and showed broad efficacy by demonstrating statistically significant superiority to placebo across a total of 15 prespecified primary and secondary outcome measures. Based upon these results, combined with the prior positive Phase 2/3 trial, Biohaven is moving forward with plans for regulatory submissions in the United States and other countries. Full results from this Phase 3 trial will be presented at upcoming medical conferences and/or published in peer-reviewed journals. Richard B. Lipton, M.D., Professor and Vice Chair of Neurology at the Albert Einstein College of Medicine and Director of the Montefiore Headache Center, said “Patients with migraine rate speed of onset as one of the most important aspects of an effective therapy. The data from this trial shows that intranasal zavegepant delivered impressive performance on this metric by demonstrating statistically significant pain relief within 15 minutes and return to normal function within 30 minutes. Additionally, non-oral treatments offer additional benefits for patients who experience nausea, vomiting or gastroparesis (with slow absorption). Intranasal zavegepant will be an important new treatment option for patients who require a rapid and non-oral option for acute treatment of their migraine attacks.” Zavegepant was statistically superior to placebo on the co-primary endpoints of pain freedom (24% vs 15%, p < 0.0001) and freedom from most bothersome symptom (40% vs 31%, p = 0.0012) at 2 hours. Zavegepant was superior to placebo demonstrating pain relief as early as 15 minutes (see Figure 1). Patients achieved return to normal function as early as 30 minutes after dosing (p < 0.006). The efficacy benefits of zavegepant were durable, including superiority versus placebo (p < 0.05) on: sustained pain freedom 2 to 24 hours; sustained pain freedom 2 to 48 hours; sustained pain relief 2 to 24 hours; and sustained pain relief 2 to 48 hours. Vlad Coric, M.D., Chief Executive Officer at Biohaven stated, “Intranasal zavegepant was designed to provide ultra-rapid pain relief and expand our CGRP receptor-antagonist franchise by providing patients with another important tool to combat migraine. The trial results clearly show that the performance of this formulation exceeded expectations by demonstrating superiority over placebo on pain relief at 15 minutes and return to normal function by 30 minutes. The impressive efficacy, safety and tolerability profile shown in this trial highlights the potential of zavegepant to usher in a new era of non-oral CGRP targeting migraine therapies that may transcend the traditional boundaries of older legacy intranasal migraine approaches. Biohaven is committed to delivering on its promise to provide new treatment options for the millions of people living with this debilitating disease and these data represent a major milestone in that endeavor,” (see Figure 2). The Phase 3 pivotal study is a randomized, double-blind, placebo-controlled clinical trial that randomized 1,405 adults with at least a one-year history of migraine (with or without aura) and migraine attacks lasting, on average, 4 to 72 hours if untreated. Conducted at 94 sites in the United States, the study evaluated the safety and efficacy of zavegepant intranasal spray taken as needed in a single dose compared to placebo for the acute treatment of a moderate to severe migraine attack. Zavegepant showed a favorable safety and tolerability profile among study participants that was consistent with prior clinical trial experience. The most common individual adverse event in the pivotal study reported with a frequency ≥ 5% in the zavegepant treatment arm and greater than placebo was abnormal taste (21% vs 5%). The majority of AEs were mild in intensity. Biohaven plans to file a New Drug Application (NDA) for zavegepant with the U.S. Food and Drug Administration (FDA) in 1Q 2022 and other countries thereafter. If ultimately approved, zavegepant would be the first intranasal calcitonin gene-related peptide (CGRP) receptor antagonist for the acute treatment of migraine. Zavegepant is the second clinical candidate for Biohaven after FDA-approved Nurtec® ODT (rimegepant) for the acute treatment of migraine and preventive treatment of episodic migraine in adults. About Zavegepant About Migraine CGRP Receptor Antagonism About Nurtec ODT About Biohaven Forward-Looking Statements Nurtec, Nurtec ODT and NOJECTION are registered trademarks of Biohaven Pharmaceutical Ireland DAC. Biohaven Contact: Media Contact:
SOURCE Biohaven Pharmaceutical Holding Company Ltd. | ||
Company Codes: NYSE:BHVN |