BioVie Inc. today announced encouraging data suggesting that NE3107 has an impact on improving patients’ DNA methylation profiles, potentially impacting biomakers of aging-related disease states.
CARSON CITY, Nev., Dec. 06, 2022 (GLOBE NEWSWIRE) -- BioVie Inc., (NASDAQ: BIVI) (“BioVie” or the “Company”) a clinical-stage company developing innovative drug therapies for the treatment of neurological and neurodegenerative disorders and advanced liver disease, today announced encouraging data suggesting that NE3107 has an impact on improving patients’ DNA methylation profiles, potentially impacting biomakers of aging-related disease states.
The Phase 2 Investigator-Sponsored Trial (NCT05227820) enrolled a total of 23 patients with an average age of 71.1 years in an open-label, single arm study to measure changes in cognition through verbal and visual test procedures and changes in biomarkers of Alzheimer’s Disease (AD) and inflammation that can be measured in cerebral spinal fluid, blood samples, and functional magnetic resonance imaging in patients before and after treatment with 20 mg of NE3107 twice daily for 3 months.
Dr. Sheldon Jordan (the Principal Investigator) and his team presented detailed data through posters and a platform presentation at the Clinical Trial in Alzheimer’s Disease (CTAD) annual conference, held in San Francisco, CA that ended December 2, 2022. These data showed the following among patients with MMSE>=20 (i.e., mild cognitive impairment and mild AD):
- NE3107 showed the potential to enhance cognition as measured by multiple assessment tools, including a 2.2 point improvement (p=0.0173) on the modified ADAS-Cog12 scale equating to a 21.1% (p=0.0079) change compared to baseline, a 0.11 point improvement (p=0.0416) on the Clinical Dementia Rating scale (CDR), equating to 19.4% (p=0.0416) change from baseline, and a 0.07 point improvement in the ADCOMS scale, equating to 27.4% improvement (p=0.009).
- NE3107 reduced CSF phospho-tau levels by -1.66 pg/mL (p=0.0343) and the ratio of p-tau to A42 by -0.0024 (p=0.0401)
- 18 of 22 patients with abnormal baseline scans showed improvement in one or more brain regions as seen from advanced functional MRI studies
- No drug-related adverse events were observed
NE3107’s Potential Impact on Biomakers of Aging-related Disease States
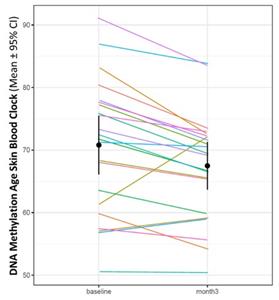
Blood samples were taken from the patients who participated in the Alzheimer’s Phase 2 trial before and after 3 months of treatment with NE3107, and these samples were analyzed to assess NE3107’s potential to reduce inflammation and alter DNA methylation associated with epigenetic biological clocks.
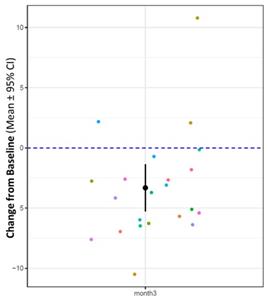
The resulting data for patients treated with NE3107 for three months showed a reduction of 3.3 years (p=0.0021) on the Horvath DNA methylation SkinBlood clock. Furthermore, 19 out of the 22 patients experienced this reduction in the SkinBlood clock score.
The finding that NE3107 affects the SkinBlood clock provides an impetus to explore further the relationship between NE3107 improving neurodegeneration and other inflammation-driven diseases.
“This additional finding from study of NE3107 in the treatment of dementia reflects a hypothesis-driven effort to investigate the effect of epigenetic change associated with an anti-inflammatory compound, as inflammation is hypothesized to drive both neurodegeneration and aging,” explained Dr. Sheldon Jordan, the trial’s Principal Investigator. “The study was designed to decrease inflammation and measure the effect using the epigenetic clocks. I am collaborating with Dr. Horvath’s team on this effort since they are the recognized leaders in the field.”
“We are very excited by this initial finding and recognize that there is still much more to learn about NE3107’s impact on the biomarkers of aging-related disease states and how this may affect disease progression,” commented Cuong Do, BioVie’s President and CEO. “We look forward to working with collaborators to answer questions such as whether the reversal will accumulate with longer treatment duration, the durability of the effect, and whether this translates to a reduced risk in diseases associated with aging.”
Epigenetics is the study of how behavior (e.g., diet, exercise) and environment affect the way our genes work in addition to the genetic code itself, and there’s an increasing body of evidence that signs of aging are epigenetic in nature.1,2 Perhaps the most studied area of epigenetics involves DNA methylation, which is the process of how methyl groups are added or removed from DNA and thus regulate the expression of various genes in our bodies.3 Many studies have shown that genes become over- or under-methylated as we age,4,5,6 thereby suggesting that the modulation of DNA methylation could enable the up- or down-regulation of specific genes and thus modulate the aging process. Dr. Steven Horvath, Professor of Human Genetics at the UCLA David Geffen School of Medicine and Professor of Human Genetics & Biostatistics at the UCLA Field School of Public Health, is a leading authority on the study of DNA methylation, and his research publications have been cited nearly 90,000 times in peer-reviewed articles. Dr. Horvath studied a large number of datasets comprised of most tissue and cell types to create the first epigenetic clock (or “biological clock” to measure “biological age”) to measure the cumulative methylation of selected sites in the genome and how this could differ from a person’s “chronological age.”
This finding that NE3107 may affect DNA methylation would be consistent with the understanding of its mechanism of action. NE3107 has been shown to modulate the expression of TNF, which is considered to be the master regulator of inflammation.7 Inflammation has been shown to be associated with hypermethylation of DNA,8 which in turn has been shown to impact a wide range of diseases, including various forms of cancers,9 age-related cognitive impairment and dementia,10 Parkinson’s disease,11 cardiovascular disease,10,12 COPD and respiratory disease,13 chronic kidney disease,14 inflammatory bowel disease,15 sepsis,16 and many others.
Contributory Explanation for Alzheimer’s and Parkinson’s Findings
Patients treated with NE3107 demonstrated enhanced cognition as evidenced by improvements on multiple assessment scales in this Phase 2 study, and the Company’s confirmatory double-blind, randomized controlled study involving subjects who have mild to moderate Alzheimer’s disease is expected to read out in 3Q2023. Patients also saw improvements in their inflammation (i.e., reduction in TNF) in a manner that is correlated with improvements in cognition, reduction of CSF phospho-tau levels and the ratio of p-tau to A42, and improvements in neuronal health as seen from functional MRI studies.
“One limitation of the AD Phase 2 trial is that its open label design limits its ability to isolate placebo effects,” commented Joseph Palumbo, BioVie’s Chief Medical Officer. “The observation that 19 out of 22 patients saw a reversal of their biological clock would suggest that there could be more going on than just a placebo effect, which provides greater confidence that the improvement in cognition is indeed based on NE3107’s impact. The biomarker and imaging improvements is also consistent with the improvements in cognition observed. We await readout from the Phase 3 trial next year to confirm these findings”
The Company announced topline results from its NM201 double-blind, placebo-controlled, proof of concept study (NCT05083260) in Parkinson’s disease (PD) on December 5, 2022 showing that symptomatic patients treated with NE3107 in addition to their stable dose of levodopa (and other Parkinson’s therapies) showed superior motor control compared to levodopa alone (trend level). Patients treated with NE3107/levodopa saw improvements of UPDRS part 3 (motor) score on Day 28 compared to Day 0 that is 3+ points better than those treated with levodopa alone at the 2 and 3 hour marks. Furthermore, patients under 70 years of age treated with NE3107/levodopa scored roughly 6 points superiority compared to levodopa alone. This level of improvement is considered by PD experts to be clinically meaningful.
“I believe this encouraging finding that NE3107 may affect the biological makers associated with aging helps explain the results we have seen from our Alzheimer’s and Parkinson’s trials,” commented Cuong Do. “NE3107 has shown the ability to modulate inflammation and insulin resistance by reducing TNF, which leads to improvements in a series of downstream factors such as metabolic dyshomeostasis, apoptosis, oxidative stress, among others. NE3107’s impact on DNA methylation helps explain the magnitude and rapid pace of impact we see in the AD and PD trials.”
About Inflammation and NE3107’s Mechanism of Action
Neuroinflammation, insulin resistance, and oxidative stress are common features in the major neurodegenerative diseases, including Alzheimer’s Disease (AD), Parkinson’s Disease (PD), frontotemporal lobar dementia, and ALS. NE3107 is an oral small molecule, blood-brain permeable, compound with potential anti-inflammatory, insulin sensitizing, and ERK-binding properties that may allow it to selectively inhibit ERK-, NFκB- and TNF-stimulated inflammation. NE3107’s potential to inhibit neuroinflammation and insulin resistance forms the basis for the Company’s work testing the molecule in AD and PD patients.
Remarkable parallels exist between AD and PD, among them activated microglia driving inflammation, involvement of TNFα, oxidative stress, mitochondrial dysfunction, and insulin resistance. In preclinical and clinical studies, NE3107 reduced inflammation and enhanced insulin sensitivity, both of which are important to PD pathology. Preclinical studies in marmoset monkeys have shown NE3107 administered alone to be as pro-motoric as levodopa, underscoring the apparently critical role of inflammation in expression of PD dysmobility. When NE3107 was administered with levodopa, the combination improved motor control better than either drug alone. Furthermore, in the marmoset study, NE3107 reduced the severity of levodopa induced dyskinesia (LID) concurrent with pro-motoric benefit and decreased neurodegeneration, preserving twice as many dopaminergic neurons compared to control.
About BioVie
BioVie Inc. (NASDAQ: BIVI) is a clinical-stage company developing innovative therapies to overcome unmet medical needs in chronic debilitating conditions. In neurodegenerative disease, the Company’s drug candidate NE3107 inhibits inflammatory activation of ERK and NFkB (e.g., TNF signaling) that leads to neuroinflammation and insulin resistance, but not their homeostatic functions (e.g., insulin signaling and neuron growth and survival). Both are drivers of Alzheimer’s and Parkinson’s diseases. The Company is conducting a potentially pivotal Phase 3 randomized, double blind, placebo controlled, parallel group, multicenter study to evaluate NE3107 in patients who have mild to moderate Alzheimer’s disease (NCT04669028) and is targeting primary completion in mid-2023. An estimated six million Americans suffer from Alzheimer’s. A Phase 2 study of NE3107 in Parkinson’s disease (NCT05083260) has completed and provided its topline data readout in December 2022. In liver disease, the Company’s Orphan drug candidate BIV201 (continuous infusion terlipressin), with FDA Fast Track status, is being evaluated in a US Phase 2b study for the treatment of refractory ascites due to liver cirrhosis with top-line results anticipated in mid-2023. BIV201 is administered as a patent-pending liquid formulation. The active agent is approved in the U.S. and in about 40 countries for related complications of advanced liver cirrhosis. For more information, visit http://www.bioviepharma.com/.
Forward-Looking Statements
This press release contains forward-looking statements, which may be identified by words such as “expect,” “look forward to,” “anticipate” “intend,” “plan,” “believe,” “seek,” “estimate,” “will,” “project” or words of similar meaning. Although BioVie Inc. believes such forward-looking statements are based on reasonable assumptions, it can give no assurance that its expectations will be attained. Actual results may vary materially from those expressed or implied by the statements herein due to the Company’s ability to successfully raise sufficient capital on reasonable terms or at all, available cash on hand and contractual and statutory limitations that could impair our ability to pay future dividends, our ability to complete our pre-clinical or clinical studies and to obtain approval for our product candidates, to successfully defend potential future litigation, changes in local or national economic conditions as well as various additional risks, many of which are now unknown and generally out of the Company’s control, and which are detailed from time to time in reports filed by the Company with the SEC, including quarterly reports on Form 10-Q, reports on Form 8-K and annual reports on Form 10-K. BioVie Inc. does not undertake any duty to update any statements contained herein (including any forward-looking statements), except as required by law.
For Public/Press Relations Inquiries:
Dennis Consorte
(201) 344-9648
dennisconsorte@gmail.com
For Investor Relations Inquiries:
Bruce Mackle
Managing Director
LifeSci Advisors, LLC
bmackle@lifesciadvisors.com
1 Oberdoerffer P Nat Rev Mol Cell Biol 2007, 8:692–702.
2 Campisi J Gerontol A Biol Sci Med Sci 2009, 64A:175–178.
3 Moore L Neuropsychopharmacology vol 38, pages23–38 (2013)
4 Fraga MF Ann N Y Acad Sci 2007, 1100:60–74.
5 Christensen BC PLoS Genet 2009, 5:e1000602
6 Horvath S Genome Biol 2012, 13:R97
7 Jang et al. Int J Mol Sci. 2021 Mar; 22(5): 2719
8 Stenvinkel P doi: 10.1111/j.1365-2796.2007.01777.x
9 Wang Z Nucleic Acids Research, 2020, Vol. 48, No. 5
10 Sugden K Neurology 2022;99:e1402-e1413
11 Tang X DOI: 10.1002/mds.29157
12 Tabaeia S Artificial Cells, Nanomedicine, and Biotechnology, 47:1, 2031-2041
13 Qiu W Am J Respir Crit Care Med Vol 185, Iss. 4, pp 373–381, Feb 15, 2012
14 Rysz C Int. J. Mol. Sci. 2022, 23(13), 7108
15 Kraiczy J Mucosal Immunology volume 9, pages 647–658 (2016)
16 Rump K Sci Rep 9, 18511 (2019)
Photos accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/93789285-11cc-468b-b03e-8e30f4d1e0f4
https://www.globenewswire.com/NewsRoom/AttachmentNg/89a10cb4-cf92-4bd3-99c5-e3593d48e60e