ClearPoint Neuro, Inc. today congratulates its partner PTC Therapeutics on completion of its BLA submission to the U.S. Food and Drug Administration (FDA) for the approval of Upstaza.
SOLANA BEACH, Calif., March 19, 2024 (GLOBE NEWSWIRE) -- ClearPoint Neuro, Inc. (Nasdaq: CLPT) (the “Company”), a global device, cell, and gene therapy-enabling company offering precise navigation to the brain and spine, today congratulates its partner PTC Therapeutics, Inc. on completion of its BLA submission to the U.S. Food and Drug Administration (FDA) for the approval of Upstaza™ (eladocagene exuparvovec), an investigational treatment for AADC Deficiency. If approved, Upstaza™ would become the first therapy to treat AADC Deficiency in the United States.
“AADC Deficiency is a devastating rare pediatric movement disorder that causes significant developmental delays and autonomic symptoms starting from birth. Patients with AADC deficiency are at a high risk of death in the first decade of life,” stated Jeremy Stigall, Chief Business Officer at ClearPoint Neuro. “The Upstaza™ BLA is the first filing for FDA approval of a treatment that addresses this devastating condition. Through our partnership with PTC, we are demonstrating our commitment to drive progress for the AADC Deficiency community.”
About aromatic L-amino acid decarboxylase (AADC) deficiency
AADC deficiency is a fatal, rare genetic disorder that typically causes severe disability and suffering from the first months of life, affecting every aspect of life – physical, mental and behavioral. The suffering of children with AADC deficiency may be exacerbated by episodes of distressing seizure-like oculogyric crises causing the eyes to roll up in the head, frequent vomiting, behavioral problems, and difficulty sleeping.
The lives of affected children are severely impacted and shortened. Ongoing physical, occupational and speech therapy, and interventions, including surgery, also are often required to manage potentially life-threatening complications such as infections, severe feeding and breathing problems.
About the SmartFlow® Cannula
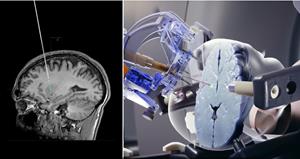
With over 7,000 cannulas sold to date, SmartFlow is the only co-labeled device to gain approval by a regulatory agency for delivery of an approved gene therapy to the brain. The industry-leading cannula is used by many of ClearPoint Neuro’s 50+ pharmaceutical, academic, and biotech partners to bypass the blood brain barrier and deliver therapeutics to regions of interest using Convection Enhanced Delivery (CED) under direct image guidance. The SmartFlow Cannula has 510(k) clearance from the FDA for use in the United States for the aspiration of cerebrospinal fluid or injection of the chemotherapy drug Cytarabine into the ventricles. It has also been CE marked to deliver approved fluids into the brain and for aspiration of cerebrospinal fluid, and holds regulatory clearance for clinical use in Israel and Brazil. SmartFlow is utilized in approved clinical and preclinical studies for various research and drug trials.
About ClearPoint Neuro
ClearPoint Neuro is a device, cell, and gene therapy-enabling company offering precise navigation to the brain and spine. The Company uniquely provides both established clinical products as well as pre-clinical development services for controlled drug and device delivery. The Company’s flagship product, the ClearPoint Neuro Navigation System, has FDA clearance and is CE-marked. ClearPoint Neuro is engaged with healthcare and research centers in North America, Europe, Asia, and South America. The Company is also partnered with the most innovative pharmaceutical/biotech companies, academic centers, and contract research organizations, providing solutions for direct CNS delivery of therapeutics in pre-clinical studies and clinical trials worldwide. To date, thousands of procedures have been performed and supported by the Company’s field-based clinical specialist team, which offers support and services to our customers and partners worldwide. For more information, please visit www.clearpointneuro.com.
Forward-Looking Statements
This press release contains forward-looking statements within the context of the federal securities laws, which may include the Company’s expectation for the future market of its products and services, and other performance and results. These forward-looking statements are based on management’s current expectations and are subject to the risks inherent in the business, which may cause the Company’s actual results to differ materially from those expressed in or implied by forward-looking statements. Particular uncertainties and risks include those relating to: global and political instability, supply chain disruptions, labor shortages, and macroeconomic and inflationary conditions; future revenue from sales of the Company’s products and services; the Company’s ability to market, commercialize and achieve broader market acceptance for new products and services offered by the Company; the ability of our biologics and drug delivery partners to achieve commercial success, including their use of the Company’s products and services in their delivery of therapies; the Company’s expectations, projections and estimates regarding expenses, future revenue, capital requirements, and the availability of and the need for additional financing; the Company’s ability to obtain additional funding to support its research and development programs; the ability of the Company to manage the growth of its business; the Company’s ability to attract and retain its key employees; and risks inherent in the research, development, and regulatory approval of new products. More detailed information on these and additional factors that could affect the Company’s actual results are described in the “Risk Factors” section of the Company’s Annual Report on Form 10-K for the year ended December 31, 2023, which has been filed with the Securities and Exchange Commission. The Company does not assume any obligation to update these forward-looking statements.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/d375ef5e-925f-4976-8bb2-63f3e90871a3

Contact: Media Contact: Jacqueline Keller, Vice President of Marketing (888) 287-9109 ext. 4 info@clearpointneuro.com Investor Relations: Danilo D’Alessandro, Chief Financial Officer (888) 287-9109 ext. 3 ir@clearpointneuro.com