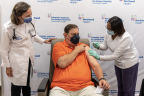
As part of a new National Institutes of Health (NIH) coronavirus disease 2019 (COVID-19) vaccine clinical trial, The Feinstein Institutes for Medical Research – the science arm of Northwell Health – delivered its first set of extra shots to eligible New York patients with an autoimmune disease.
MANHASSET, N.Y.--(BUSINESS WIRE)-- As part of a new National Institutes of Health (NIH) coronavirus disease 2019 (COVID-19) vaccine clinical trial, The Feinstein Institutes for Medical Research – the science arm of Northwell Health – delivered its first set of extra shots to eligible New York patients with an autoimmune disease.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20210902005824/en/
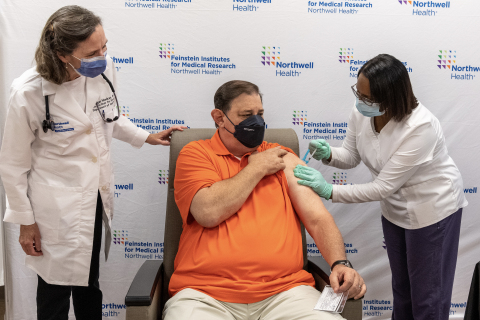
Robert Cass became the first clinical trial participant in New York living with an autoimmune disease to receive his extra COVID-19 vaccine. (Credit: Feinstein Institutes)
An estimated 8 percent of Americans have an autoimmune disease and COVID-19 has disproportionality affected this vulnerable population. The new Phase 2 trial is sponsored and funded by the National Institute of Allergy and Infectious Diseases (NIAID), part of NIH. It is called the “COVID-19 Booster Vaccine in Autoimmune Disease Non-Responders” and will study the antibody response to an extra shot of an authorized or approved COVID-19 vaccine in people with an autoimmune disease whose body did not respond to an original vaccine regimen. The trial will also look at whether holding immunosuppressive therapy around the time of the additional vaccine will improve the antibody response.
Robert Cass, a grandfather from Long Island who lives with Sjogren’s syndrome and rheumatoid arthritis, was the first patient in New York State to enroll and receive the vaccine at the Feinstein Institutes – and one of 10 trial participants to receive their extra shot on September 1. Five months after receiving his second Pfizer vaccine shot in March, antibodies were not detected in his blood.
“Like everyone else, I am eager for this pandemic to end. When I learned of the opportunity to participate in a clinical trial, I was ready to roll up my sleeve – what do I have to lose?” said Mr. Cass. “If my participation in this clinical trial can help others like me, that’s a good thing.”
The study’s primary goal is to determine the proportion of participants who have a significantly better antibody response four weeks after receiving the extra vaccine dose. Additionally, researchers will investigate whether antibody response to the vaccine is related to medications, disease and/or vaccine type, how other cells in the immune system respond to the additional dose of vaccine and whether extra vaccine doses have any effect on autoimmune disease activity.
The NIH announced the start of the trial on August 27, naming the Feinstein Institutes as one of the four national sites leading the study, along with Oklahoma Medical Research Foundation in Oklahoma City, the University of Michigan in Ann Arbor and the University of Pennsylvania in Philadelphia.
“People living with an autoimmune disease often take some form of immunosuppressive therapy, which may hinder their body’s ability to create a robust COVID-19 antibody response when given a vaccine,” said Meggan Mackay, MD, professor in the Institute of Molecular Medicine at the Feinstein Institutes and co-principal investigator on the trial. “We are eager to enroll patients in this nationwide clinical trial to study the efficacy of an additional vaccine dose to boost their immune system and help protect millions from this virus.”
The study will enroll approximately 600 participants ages 18 years and older across multiple sites nationwide, all of whom must show a negative or suboptimal antibody response to two doses of the Moderna COVID-19 vaccine, two doses of the Pfizer-BioNTech COVID-19 vaccine, or one dose of the Johnson & Johnson COVID-19 vaccine. The participants will be followed for 13 months, with preliminary results expected in November 2021. The study will initially include people with one of five autoimmune diseases: multiple sclerosis, pemphigus, rheumatoid arthritis, systemic lupus erythematosus or systemic sclerosis. All participants in the study must be taking one of three immunosuppressive therapies: mycophenolate mofetil (MMF) or mycophenolic acid (MPA); methotrexate (MTX); or B cell-depleting drugs.
“Because Dr. Mackay is an expert and leader in autoimmune disease and immunology, this NIH-supported trial in COVID-19 vaccine responses is timely and important,” said Kevin J. Tracey, MD, president and CEO of the Feinstein Institutes. “The results of these efforts will guide future efforts to save lives during the pandemic.”
Since the start of the pandemic, the Feinstein Institutes has focused its research on studying the safety and efficacy of potential COVID-19 therapies, having initiated 15 clinical trials and enrolled more than 1,700 patients. Through Feinstein’s COVID-19 Research Consortium (CRC), more than 500 clinicians, statisticians and scientists have published over 400 peer-reviewed manuscripts related to the virus in efforts to inform the public and research community.
Information about the COVID-19 Booster Vaccine in Autoimmune Disease Non-Responders trial, including the locations of study sites, is available at ClinicalTrials.gov (NCT05000216).
About the Feinstein Institutes
The Feinstein Institutes for Medical Research is the research arm of Northwell Health, the largest health care provider and private employer in New York State. Home to 50 research labs, 3,000 clinical research studies and 5,000 researchers and staff, the Feinstein Institutes raises the standard of medical innovation through its five institutes of behavioral science, bioelectronic medicine, cancer, health system science, and molecular medicine. We make breakthroughs in genetics, oncology, brain research, mental health, autoimmunity, and are the global scientific leader in bioelectronic medicine – a new field of science that has the potential to revolutionize medicine. For more information about how we produce knowledge to cure disease, visit http://feinstein.northwell.edu and follow us on LinkedIn.
View source version on businesswire.com: https://www.businesswire.com/news/home/20210902005824/en/
Source: The Feinstein Institutes for Medical Research