eCential Robotics, a French growth MedTech company that designs and produces a system unifying 2D/3D imaging, surgical navigation and robotics, today announced FDA 510(k) clearance of its 3D imaging, navigation and robotics guidance system, securing the penetration of its unified robotic platform in the United States.
|
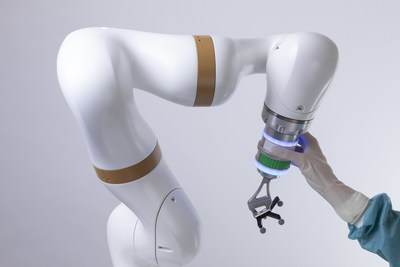
GIÈRES, France, Sept. 1, 2022 /PRNewswire/ -- eCential Robotics, a French growth MedTech company that designs and produces a system unifying 2D/3D imaging, surgical navigation and robotics, today announced FDA 510(k) clearance of its 3D imaging, navigation and robotics guidance system, securing the penetration of its unified robotic platform in the United States. After having recently concluded partnership with US implant companies, eCential Robotics aims from now on access to the North American market. Securing the U.S. market penetration plan in the mid-term Created in 2009 by Stéphane Lavallée, president and chairman, eCential Robotics' goal is to reinforce the safety and accuracy of surgical procedures, while offering simplicity and speed of use. With the objective of enabling surgeons to operate in a reliable and simple environment for the best benefit of patients, eCential Robotics has concentrated its development on a unique and innovative concept: focusing surgical workflows on the essential to make robotic-assisted bone surgery simple enough in clinical routine that it becomes a standard of care. eCential Robotics welcomes implant companies to create specific Apps for multiple indications in notably spine, cranial, traumatology, orthopaedics, sports medicine. Historically, after several years dedicated to the massive development of its unique technology and workflow, eCential Robotics obtained CE marking and successfully launched the commercialization of its first platform in France. Ten units have been sold and installed in Europe and more than two thousand surgeries performed. To support the penetration of the North American market, an eCential Robotics, Inc. subsidiary was created in December 2020. In Q1 2022, eCential Robotics conducted pre-clinical evaluation tests in the USA with neurosurgeons and orthopedic surgeons and hired its first local application engineer. FDA 510(k) clearance of the intra-operative 2D and 3D imaging, navigation and robotic guidance platform, with a first universal application in spine surgery, now secures the plan to access the North American market over the next few years. "The FDA clearance of the eCential Robotics unified platform recognizes reliability and robustness of our product, confirms the confidence in eCential Robotics' unique concept of focusing surgical workflows on the essential via a unified, open and multi-app system, and also encourages our ambition to expand our footprint in the United States", said Mrs. Laurence Chabanas, eCential Robotics Chief Strategy Officer and USA CEO. "This 510(k) is fundamental to our strategy. We are excited about these bold new and disruptive technologies and the role that eCential Robotics can play in reshaping bone surgical procedures and restoring patients' quality of life," she added. The eCential Robotics team will be exhibiting at SMISS 2022 and NASS 2022 congresses in the U.S. and will be demonstrating its robotic arm. The only available solution fully unified In its core design, the surgical platform of eCential Robotics unifies intraoperative 2D/3D imaging, navigation, and robotics. As the only available solution that is fully unified, it avoids the pitfalls of traditional image-navigation pairing, such as unreliable calibration and registration steps, and streamlines the surgical workflow by automating numerous technical steps. A single user interface for all devices enables to control the imaging, navigation and robotic functionalities from a single input and output graphical display screen. The system is also a fully open solution, meaning that it can be used with any manufacturer's implants. Built around a range of applications ("Apps") currently dedicated to spine surgery, the eCential Robotics platform will in the future expand to multiple bone surgery indications. The unified open platform is a medical device that provides 2D/3D medical imaging and stereotaxic guidance. The system consists of three mobile interconnected units: a mobile C-arm, a mobile viewing workstation, and a mobile collaborative robot. The mobile X-ray system is an imaging robot with 5-axis supporting 2D imaging and 3D imaging of anatomical structures and high contrast objects. The navigation feature with both freehand navigation and robotic guidance, is based on the standard and established technique of navigation systems using 3D optical position tracking technology, including a stereoscopic camera, the computer platform with monitors, a navigation software, a robotic arm and instruments equipped with marker spheres to enable an exact localization in space. "With still a huge potential for innovation, eCential Robotics simplifies the entire workflow for a seamless surgical experience," declared David Armand, eCential Robotics CTO. About eCential Robotics eCential Robotics is a Grenoble-based company specializing in surgical robotics. It develops and markets a unique system unifying 2D/3D robotic imaging and real-time navigation. With 80 patents and seven trademarks, it pursues a disruptive innovation strategy. It offers orthopedic and neurosurgeons easy-to-use, cutting-edge technology to visualize their operations, particularly minimally invasive surgery. The eCential Robotics platform is a universal system open to all implants. Winner of the Bpifrance Worldwide Innovation Challenge in 2018, the company designs and produces all its equipment in Grenoble, France, including hardware and software. Please visit www.ecential-robotics.com and follow us on LinkedIn (eCential Robotics) and Twitter (@ecentialrobot). Media contact Investor relations Marie CABRIÈRES Benjamin COLAS Photo - https://mma.prnewswire.com/media/1888885/ECENTIAL_ROBOTICS.jpg
SOURCE ECENTIAL ROBOTICS |