Biohaven Pharmaceutical Holding Company Ltd. (NYSE: BHVN), today announced that the U.S. Food and Drug Administration (FDA) has approved NURTEC® ODT (rimegepant 75 mg) for the preventive treatment of migraine
|
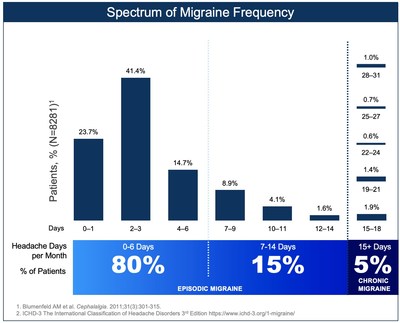
NEW HAVEN, Conn., May 27, 2021 /PRNewswire/ -- Biohaven Pharmaceutical Holding Company Ltd. (NYSE: BHVN), today announced that the U.S. Food and Drug Administration (FDA) has approved NURTEC® ODT (rimegepant 75 mg) for the preventive treatment of migraine. NURTEC® ODT is indicated for adult patients with episodic migraine, e.g. those who experience less than 15 headache days per month. The approved product label was also expanded to include the use of NURTEC ODT 75 mg up to 18 doses/month, allowing for both acute and preventive therapy in the same patient. This new approval makes NURTEC ODT the first oral CGRP antagonist approved for the preventive treatment of migraine, and the only migraine medication approved as a dual therapy for both the acute and preventive treatment. NURTEC ODT is approved for acute treatment in all eligible adult patients with migraine, regardless of the number of monthly migraine days. Since approximately 95% of all U.S. migraine patients experience less than 15 headache days per month, the new indication of preventive treatment significantly expands the market potential of NURTEC ODT and provides a new preventive treatment option for the vast majority of people living with migraine. Peter J. Goadsby, M.D., Ph.D., D.Sc., Professor of Neurology at the University of California, Los Angeles and King's College London, recipient of the 2021 Brain Prize for his groundbreaking research discovering the role of CGRP in migraine, and co-author of the Phase 3 preventive study published in The Lancet, commented, "The FDA approval of NURTEC ODT for the preventive treatment of migraine--along with its acute treatment indication--is one of the most groundbreaking things to happen to migraine treatment in my 40 years of practicing headache medicine. To have one medication patients can use to treat and prevent migraine will likely change the treatment paradigm for many of the millions of people who live with migraine." NURTEC ODT, with its novel quick-dissolve tablet formulation, works by blocking the CGRP receptor, treating a root cause of migraine. With this targeted mechanism of action, NURTEC ODT provides a more complete, flexible treatment plan that gives people with migraine increased control of their disease. A single dose of NURTEC ODT can deliver fast pain relief that lasts up to 48 hours for many patients. NURTEC ODT can be taken up to once daily as needed to stop migraine attacks or taken every other day to help prevent migraine and reduce the number of monthly migraine days. This approval represents an important advancement in care for the several hundreds of millions of people living with migraine across the globe. For the first time, one medication can treat and prevent migraine attacks. Vlad Coric, M.D., Chief Executive Officer of Biohaven noted, "This FDA approval marks the beginning of a new era for migraine treatments, allowing the potential for healthcare professionals to prescribe, and patients to have, a single medication to treat and prevent migraine attacks. NURTEC ODT is dissolving the line between acute and preventive treatment. This groundbreaking approach to treating the full spectrum of migraine disease, from acute therapy to prevention, can have a significant impact in a patient's life by helping to decrease treatment plan complexity and reduce challenges with adherence and polypharmacy. At Biohaven, neuroinnovation is at the center of our focus and we are committed to finding and advancing paradigm shifting treatment options to help people with neurological diseases so they can live life without the burden of these illnesses." The FDA approval of NURTEC ODT is based on a double-blind, randomized, placebo-controlled Phase 3 clinical trial with an open label extension. Primary study endpoint results demonstrated that NURTEC was superior to placebo, decreasing monthly migraine days by 4.3 days/month after three months of treatment. The preventive effects of NURTEC were seen as early as the first week of therapy. Further, a key secondary endpoint result showed that approximately half of NURTEC-treated patients had a 50% or greater reduction in the number of moderate-to-severe migraine days per month. Carolyn Armitage, a NURTEC preventive clinical trial participant shared, "As a middle and high school teacher, I know how important it is to show up every day for my students. I value the opportunity my career provides me to help shape young minds and lives. But having a migraine attack almost three times a week has had a devastating impact on my professional and personal life. I wasn't able to be present for my students, family or friends. After trying NURTEC ODT through the clinical trial, I'm so pleased that the frequency and severity of my migraine have subsided making them more manageable." In a pivotal trial for the preventive treatment of migraine, NURTEC was generally well tolerated with the most common side effects being nausea (2.7% vs 0.8% in placebo) and stomach pain/indigestion (2.4% vs. 0.8% in placebo). NURTEC ODT is contraindicated in patients with a history of hypersensitivity to rimegepant, NURTEC ODT, or to any of its components. A recent survey, Preventing Migraine Attacks: A Current Perspective by the National Headache Foundation confirms that 84 percent of people currently taking a preventive treatment wish there was a better treatment option. Among respondents, 53% wanted more migraine free days and 67% reported that their risk of anxiety and depression increases as the number of migraine attacks increase. Nearly all respondents (98%) reported they are willing to consider a new oral treatment. Biohaven Patient Access Commitment Biohaven is committed to supporting the migraine community by eliminating barriers to medication access. The company plans to continue the $0 co-pay card in which eligible people will pay as little as $0. For eligibility requirements and to enroll in the patient support program, please call 1-833-4-NURTEC or visit www.NURTEC.com. About NURTEC ODT Indication Important Safety Information Before you take NURTEC ODT, tell your healthcare provider (HCP) about all your medical conditions, including if you:
Tell your HCP about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. NURTEC ODT may cause serious side effects including allergic reactions, including trouble breathing and rash. This can happen days after you take NURTEC ODT. Call your HCP or get emergency help right away if you have swelling of the face, mouth, tongue, or throat or trouble breathing. This occurred in less than 1% of patients treated with NURTEC ODT. The most common side effects of NURTEC ODT were nausea (2.7%) and stomach pain/indigestion (2.4%). These are not the only possible side effects of NURTEC ODT. Tell your HCP if you have any side effects. You are encouraged to report side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1–800–FDA–1088 or report side effects to Biohaven at 1–833–4NURTEC. See full Prescribing Information and Patient Information. About Migraine About CGRP Receptor Antagonism About Biohaven Forward-looking Statement NURTEC and NURTEC ODT are registered trademarks of Biohaven Pharmaceutical Ireland DAC. Neuroinnovation is a trademark of Biohaven Pharmaceutical Holding Company Ltd. Biohaven Contact Media Contact
SOURCE Biohaven Pharmaceutical Holding Company Ltd. |
||
Company Codes: NYSE:BHVN |