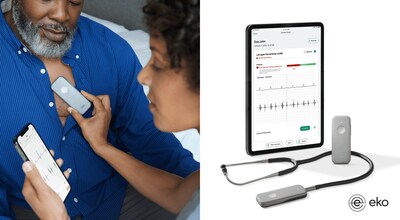
Eko Health announces FDA clearance for its Low EF detection AI. For the first time, U.S. healthcare providers can now detect Low EF, a key heart failure indicator, in 15 seconds using an Eko stethoscope during a routine physical examination.
|
Developed with Mayo Clinic, Eko Health's Low Ejection Fraction (Low EF) AI gives healthcare professionals a powerful tool to more accurately assess possible heart failure in at-risk patients during a standard physical exam SAN FRANCISCO, April 2, 2024 /PRNewswire/ -- Eko Health, a pioneer in applying artificial intelligence (AI) for early detection of heart and lung diseases, announces FDA clearance for its Low EF detection AI. For the first time, U.S. healthcare providers can now detect Low EF, a key heart failure indicator, in 15 seconds using an Eko stethoscope during a routine physical examination. This leap in early detection marks both a significant medical innovation and a new era in the detection of cardiovascular disease. In the U.S., more than 6 million people battle heart failure, with half of them experiencing heart failure with reduced ejection fraction (HFrEF)—a condition marked by the heart's inability to pump blood effectively.1 Traditional heart failure detection tools, such as echocardiography, are often unavailable in primary care settings as they are costly, require specialized training, and add significant time. As a result, many heart failure cases go undiagnosed until symptoms force a specialist or emergency hospital visit, leading to worse patient outcomes and exacerbated healthcare costs.2 Eko's Low EF AI disrupts this status quo by embedding rapid and accessible low ejection fraction detection into a stethoscope exam on the front lines of care. "The ability to identify a hidden, potentially life-threatening heart condition using a tool that primary care and subspecialist clinicians are familiar with – the stethoscope – can help us prevent hospitalizations and adverse events," said Dr. Paul Friedman, Chair of the Department of Cardiovascular Medicine at Mayo Clinic in Rochester. "Importantly, since a stethoscope is small and portable, this technology can be used in urban and remote locations, and hopefully help address care in underserved areas." The Low EF AI will be added to Eko's SENSORA Cardiac Early Detection Platform, the latest advancement to the platform which already features FDA-cleared algorithms to identify AFib and structural heart murmurs, often an indicator of valvular heart disease. When Low EF is detected in a primary care exam with SENSORA, access to life-extending treatment can be expedited with a referral to the cardiology department for thorough diagnostic testing and treatment evaluation. "The stethoscope, the most recognizable symbol of healthcare, touches the lives of an estimated one billion people around the globe every year," said Connor Landgraf, co-founder & CEO of Eko Health. "With Eko's Low EF AI, we've transformed the icon of medicine into an AI-powered heart failure early detection tool that can help improve access to care for millions of patients, at a fraction of the time and cost of echocardiography. It's been a privilege to work alongside Mayo Clinic in this groundbreaking endeavor." Clinical Development & Validation Highlights:
About Eko Health For more information visit www.ekohealth.com. Mayo Clinic and Dr. Friedman have a financial interest in the technology referenced in this news release. Mayo Clinic will use any revenue it receives to support its not-for-profit mission in patient care, education and research. References:
Media Contact:
SOURCE Eko Health |