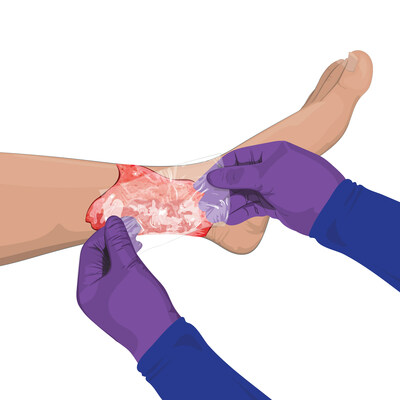
Convatec has received clearance from the U.S. Food and Drug Administration for its InnovaBurn® placental extracellular matrix medical device for management of complex surgical wounds and burns, including partial-thickness second-degree burns.
| Convatec technology is only medical device of its kind MEMPHIS, Tenn., March 2, 2023 /PRNewswire/ -- Convatec has received clearance from the U.S. Food and Drug Administration for its InnovaBurn® placental extracellular matrix medical device for management of complex surgical wounds and burns, including partial-thickness second-degree burns. FDA clears first placental extracellular matrix medical device for management of second-degree burns Each year, almost 500,000 people seek medical treatment for burn injuries, according to the American Burn Association’s ameriburn.org, which cites the most recent estimates available from the Centers for Disease Control and Prevention. Of these patients, about 40,000 were hospitalized for their burn injuries. The InnovaBurn® medical device joins Convatec’s InnovaMatrix® platform as the first and only placental-derived medical devices designed for management of burns, complex surgical wounds and hard-to-heal wounds. Manufactured with Convatec’s proprietary TriCleanse™ Process, InnovaMatrix® products, including the new InnovaBurn® ECM, give physicians a next-generation medical device that preserves the inherent benefits of the placenta along with the reliability, reproducibility, and safety of a medical device. InnovaBurn® is commercially available. “InnovaBurn® is an innovative advancement in ECM technology for patients with partial-thickness, second-degree burns,” said Debra Noble, sales director of Convatec’s U.S. Burn Division. “InnovaBurn® allows burn patients the benefits of the latest and best placental ECM technology. Our unique source material helps us to manufacture some of the largest ECM sizes in the industry much more affordably, so more patients have access to this brand new technology. Physicians tell us they appreciate the special sizing and InnovaBurn’s ease of use that help them manage larger wounds like burns.” Indicated for the management of wounds, InnovaBurn® is a xenograft controlled for genetic variability and environmental and lifestyle factors, including diet and activity levels. The device also can be used on traumatic lacerations, dehisced incisional wounds, pressure and venous ulcers, post-Mohs surgical wounds, post-surgical incisions, and diabetic ulcers. InnovaBurn® requires no preparation, no specific placement orientation, no tissue tracking and no special storage. It conforms and adheres to the wound site. InnovaMatrix® products are manufactured with Convatec’s proprietary TriCleanse™ Placental Extracellular Matrix Process, which thoroughly decellularizes the ECM, disinfects the tissue and deactivates viruses while maintaining the ECM’s structural proteins that can help with healing. This creates a clean, efficient matrix for host cells to bridge across and remodel the defect. InnovaBurn® was cleared by the FDA through the 510(k) medical device pathway. Medical devices are products that are reviewed by the FDA and meet specific criteria via the 510(k) or Premarket Approval pathway. FDA-cleared devices undergo rigorous review. InnovaBurn® joins the InnovaMatrix® product family, which also includes the InnovaMatrix® AC placental ECM device that is available in several sizes; InnovaMatrix® FS, the fenestrated form designed for wounds with heavy exudate; and InnovaMatrix® PD, the particulate form. Convatec’s Advanced Tissue Technologies, formerly Triad Life Sciences, focuses on regenerative medicine and developing biologically derived innovative products to address unmet clinical needs in surgical wounds, chronic wounds and burns. Regenerative medicine and biologically derived therapies are frequently used to treat hard-to-heal wounds, which affect about 8.2 million Medicare patients each year in the U.S., according to Advances in Wound Care’s May 2021 publication ‘Human wound and its burden: Updated 2020 Compendium of Estimates.’ Founded in 2017, Triad Life Sciences, based in Memphis, Tennessee, was acquired in 2022 by Convatec Group Plc. For more information about InnovaMatrix® products, visit www.triadls.com and follow on LinkedIn @ConvatecWound, on Twitter @ConvatecWoundUS and on Facebook @ConvatecInc. Convatec Group Plc Pioneering trusted medical solutions to improve the lives we touch: Convatec is a global medical products and technologies company, focused on solutions for the management of chronic conditions, with leading positions in advanced wound care, ostomy care, continence and critical care, and infusion care. With around 10,000 colleagues, and a promise to be forever caring, our products and services are available in over 100 countries. Our solutions provide a range of benefits, from infection prevention and protection of at-risk skin, to improved patient outcomes and reduced care costs. The company is a constituent of the FTSE 100 Index (LSE: CTEC) and in 2021 revenues were over $2 billion. To learn more about Convatec, please visit http://www.convatecgroup.com. MKT-2022-0152E V01 AP-60801-USA-ENGU-v1 (v1.1)
SOURCE ConvaTec | ||
Company Codes: LSE:CTEC, OTC-PINK:CNVVY |