JERSEY CITY, N.J., Aug. 8, 2017 /PRNewswire/ -- Mitsubishi Tanabe Pharma America, Inc., today announced RADICAVA (edaravone), an intravenous therapy indicated for all adult patients diagnosed with amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig's disease, is now available for treatment in the United States. RADICAVA, the first FDA-approved ALS treatment option in more than 20 years, has been demonstrated to slow the decline in the loss of physical function in ALS patients by 33 percent in its clinical trial.1,2
Experience the interactive Multichannel News Release here: https://www.multivu.com/players/English/8149851-mtpa-radicava-launch/
"It gives me great joy that RADICAVA is now available in the U.S.," said Atsushi Fujimoto, President, Mitsubishi Tanabe Pharma America. "After two decades without a new treatment, people with ALS finally have access to a new clinically meaningful treatment option for this horrible, progressive and incurable disease."
RADICAVA is given to patients through an IV and can be administered at an ALS center, physician's office, free-standing infusion center, hospital outpatient department or through a home infusion provider, depending on individuals' health plan and their physicians' determination.
"After 13 years of clinical research and investment, we have reached a seminal moment, which may shift the treatment paradigm for this terrible disease," said Tom Larson, Chief Commercial Officer, Mitsubishi Tanabe Pharma America. "As of today, all across the country, conversations between ALS specialists and patients may be substantially different. We are all extremely proud and excited to be a part of bringing RADICAVA and new hope to patients in the U.S."
Access to the product and the benefits investigation process is initiated by the HCP and facilitated through the Searchlight Support hub, which provides assistance for people who are prescribed RADICAVA. A Searchlight Support care coordinator can help HCPs identify an infusion service site based on an individual patient's geographic location. Once the benefits investigation is completed, a case manager contacts the patient to explain among other things, benefits and co-pay support options for eligible patients.
"This new treatment may give hope to every person suffering from ALS, and we pray the positive result from this trial will set the tone for more therapies going forward. We all remain committed," said Jonathan S. Katz, M.D., ALS Clinic Director, Forbes Norris MDA/ALS Research and Treatment Center at California Pacific Medical Center.
ALS is a neurodegenerative disease in which the majority of patients die within two to five years of diagnosis.1,3 An estimated 5,000-6,000 Americans are diagnosed each year with ALS, an incurable disease that affects the nerve cells in the brain and spinal cord.1,4,5 Initial symptoms can be subtle at first, and it can take 12 to 14 months to be accurately diagnosed.6,7
For more information, visit www.RADICAVA.com or contact Searchlight Support at 1-844-SRCHLGT (1-844-772-4548).
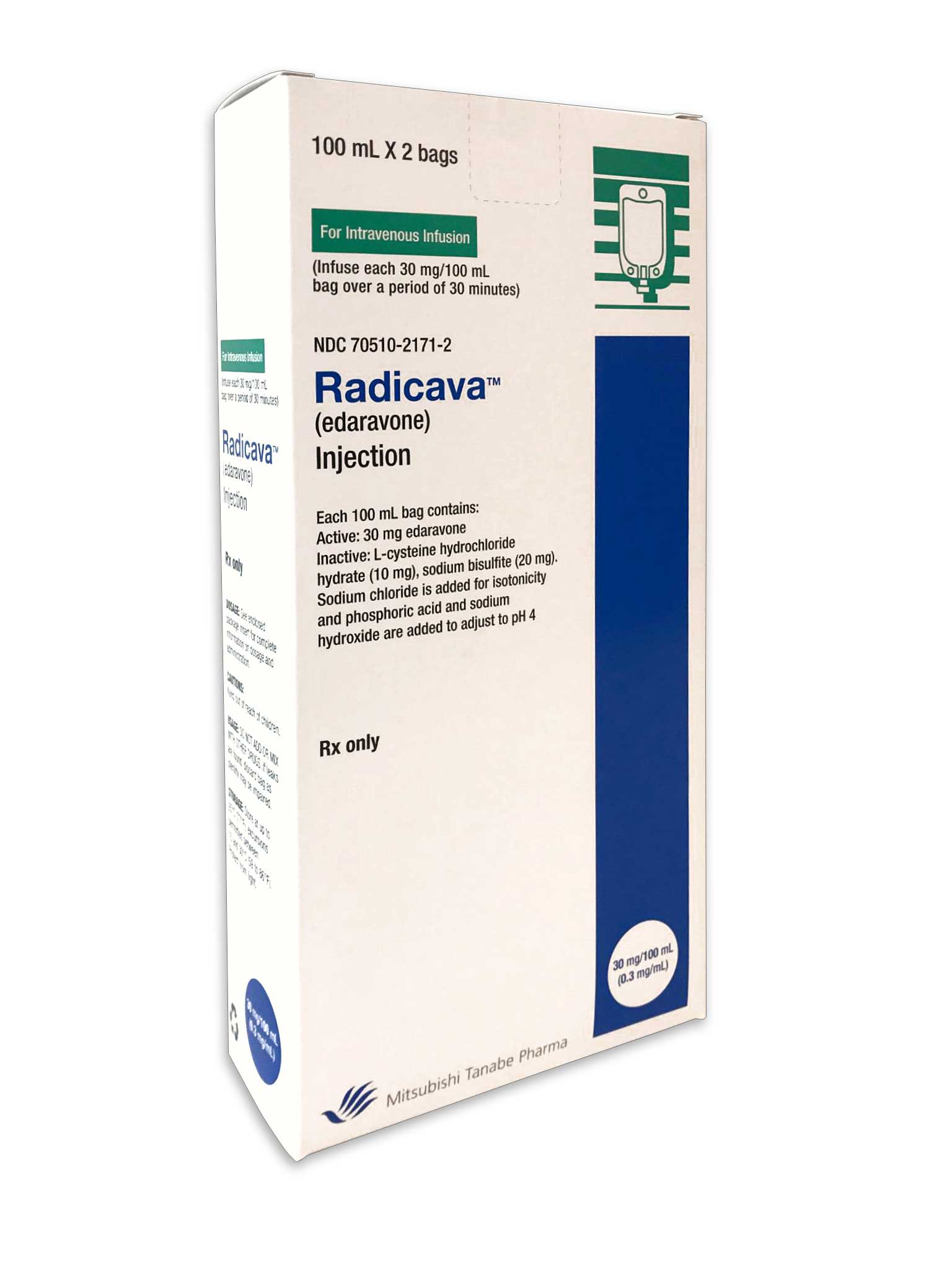
About RADICAVA (edaravone)
The U.S. Food and Drug Administration (FDA) approved RADICAVA (edaravone) on May 5, as a new treatment option indicated for all adult patients diagnosed with amyotrophic lateral sclerosis (ALS).2 In clinical trials, people given RADICAVA experienced a 33 percent lower rate of decline in the loss of physical function, compared to placebo as measured by the ALS Functional Rating Scale-Revised (ALSFRS-R), a validated rating instrument for monitoring the progression of disability in people with ALS.2,8,9
RADICAVA is administered in 28-day cycles by intravenous infusion. It takes 60 minutes to receive each 60 mg dose. For the initial cycle, the treatment is infused daily for 14 consecutive days, followed by a two-week drug-free period. All cycles thereafter are infused daily for 10 days (e.g., Monday through Friday and the following Monday through Friday) within a 14-day period, followed by a two-week drug-free period.2
Edaravone was discovered and developed for ALS by Mitsubishi Tanabe Pharma Corporation (MTPC) and commercialized in the U.S. by Mitsubishi Tanabe Pharma America. MTPC group companies began researching ALS in 2001 through a comprehensive clinical platform over a 13-year period. In 2015, edaravone was approved for use as a treatment for ALS in Japan and South Korea.
IMPORTANT SAFETY INFORMATION
Before you receive RADICAVA, tell your healthcare provider about all of your medical conditions, including if you:
- have asthma.
- are allergic to other medicines.
- are pregnant or plan to become pregnant. It is not known if RADICAVA will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if RADICAVA passes into your breast milk. You and your healthcare provider should decide if you will receive RADICAVA or breastfeed.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
What are the possible side effects of RADICAVA?
- RADICAVA may cause serious side effects including hypersensitivity (allergic) reactions and sulfite allergic reactions.
- Hypersensitivity reactions have happened in people receiving RADICAVA and can happen after your infusion is finished.
- RADICAVA contains sodium bisulfite, a sulfite that may cause a type of allergic reaction that can be serious and life-threatening. Sodium bisulfite can also cause less severe asthma episodes in certain people. Sulfite sensitivity can happen more often in people who have asthma than in people who do not have asthma.
- Tell your healthcare provider right away or go to the nearest emergency room if you have any of the following symptoms: hives; swelling of the lips, tongue, or face; fainting; breathing problems; wheezing; trouble swallowing; dizziness; itching; or an asthma attack (in people with asthma).
- Your healthcare provider will monitor you during treatment to watch for signs and symptoms of all the serious side effects.
The most common side effects of RADICAVA include bruising (contusion), problems walking (gait disturbance), and headache.
These are not all the possible side effects of RADICAVA. Call your healthcare provider for medical advice about side effects. You may report side effects to Mitsubishi Tanabe Pharma America, Inc. at 1-888-292-0058 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
For more information, including full Prescribing Information and Patient Information, please visit www.RADICAVA.com.
About Mitsubishi Tanabe Pharma America, Inc.
Based in Jersey City, N.J., Mitsubishi Tanabe Pharma America (MTPA) is a wholly-owned subsidiary of Mitsubishi Tanabe Pharma Corporation's (MTPC) 100 percent owned U.S. holding company, Mitsubishi Tanabe Pharma Holdings America, Inc. MTPA is dedicated to delivering innovative products that address the unmet medical needs of patients in the U.S. It was established by MTPC to commercialize approved pharmaceutical products in the U.S. with plans to expand its product line through collaborations with partners. For more information, please visit www.mt-pharma-america.com or follow us on Twitter at https://twitter.com/MTPharmaUS.
Overview of Mitsubishi Tanabe Pharma Corporation
Mitsubishi Tanabe Pharma, which was founded in 1678, has its headquarters in Doshomachi, Osaka, which is the birthplace of Japan's pharmaceutical industry. With business centered on ethical pharmaceuticals, Mitsubishi Tanabe Pharma is a well-established company and has the longest history of any listed company in Japan.10 In accordance with the corporate philosophy of "contributing to the healthier lives of people around the world through the creation of pharmaceuticals," the Company formulated the key concept of Open Up the Future under the Medium-Term Management Plan 16-20. Through the discovery of drugs that address unmet medical needs, centered on its priority disease areas autoimmune diseases, diabetes and kidney diseases, central nervous system diseases, and vaccines Mitsubishi Tanabe Pharma will strive to contribute to the health of patients around the world. MTPC is the parent company of MTPA and the license holder of RADICAVA. For more information, go to http://www.mt-pharma.co.jp/.
Media inquiries:
Sara Baker
212-849-9474
Sara.Baker@inventivhealth.com
Debbie Etchison
908-340-8578
Media_MTPA@mt-pharma-us.com
- National Institute of Neurological Disorders and Stroke. Amyotrophic Lateral Sclerosis (ALS) Information Page.
https://www.ninds.nih.gov/disorders/all-disorders/amyotrophic-lateral-sclerosis-als-information-page. Accessed July 2017. - RADICAVA U.S. Prescribing Information. May 2017.
- Mehta P, Kaye W, Bryan L, et al. (2016). Prevalence of Amyotrophic Lateral Sclerosis United States, 20122013. MMWR Surveill Summ, 65(8), 1-12. http://dx.doi.org/10.15585/mmwr.ss6508a1.
- Marin B, Boumediene F, Logroscino G, et al. (2016). Variation in worldwide incidence of amyotrophic lateral sclerosis: a meta-analysis. Int J Epidemiol, 46(1), 57-74. http://dx.doi.org/10.1093/ije/dyw061.
- ALS Association. Quick Facts about ALS. http://www.alsa.org/news/media/quick-facts.html. Accessed July 2017.
- ALS Therapy Development Institute. What is ALS. http://www.als.net/what-is-als/. Accessed July 2017.
- Brooks BR. (2000). Risk factors in the early diagnosis of ALS: North American epidemiological studies. Amyotrophic Lateral Sclerosis and Other Motor Neuron Disorders, 1(1), S19-S26. http://dx.doi.org/10.1080/14660820052415871.
- Simon, N. G., Turner, M. R., Vucic, S., Al-Chalabi, A., Shefner, J., Lomen-Hoerth, C., & Kiernan, M. C. (2014). Quantifying Disease Progression in Amyotrophic Lateral Sclerosis. Annals of Neurology, 76(5), 643657. http://dx.doi.org/10.1002/ana.24273
- Abe K, Aoki M, Tsuji S, et al. (2017). Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurology. 16(7), 505-512. http://dx.doi.org/10.1016/S1474-4422(17)30115-1.
- Research by TOKYO SHOKO RESEARCH, LTD.
View original content:http://www.prnewswire.com/news-releases/first-fda-approved-treatment-for-als-in-22-years-now-available-in-us-300501050.html
SOURCE Mitsubishi Tanabe Pharma America, Inc.





