GenSight Biologics announced that recent prospective real-world data from early access programs confirm the benefit of LUMEVOQ® in patients with Leber Hereditary Optic Neuropathy from the ND4 mutation, as observed in clinical trials.
- Clinically meaningful improvement in visual acuity confirmed in a real-world setting: on-chart mean visual acuity at 9 months after injection, compared to off-chart mean at baseline
- Eyes of bilaterally treated patients improve more than those of unilaterally treated patients: +23 ETDRS letters vs. +18 ETDRS letters, with a higher responder rate (63% vs. 58%), with a similar and favorable safety profile
PARIS--(BUSINESS WIRE)-- Regulatory News:
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20240305810568/en/
(Graphic: Business Wire)
GenSight Biologics (Euronext: SIGHT, ISIN: FR0013183985, PEA-PME eligible), a biopharma company focused on developing and commercializing innovative gene therapies for retinal neurodegenerative diseases and central nervous system disorders, today announced that recent prospective real-world data from early access programs (EAP) confirm the benefit of LUMEVOQ® in patients with Leber Hereditary Optic Neuropathy from the ND4 mutation (ND4-LHON), as observed in clinical trials.
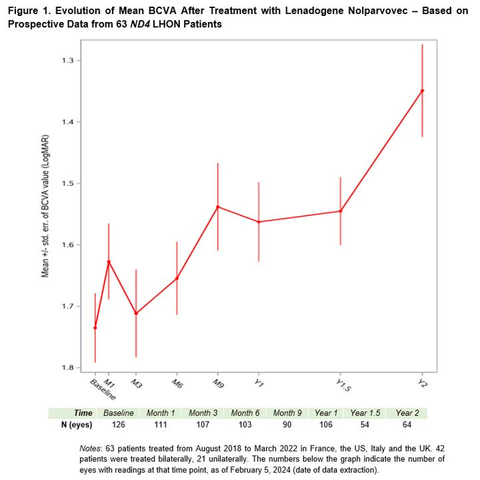
One year after treatment, eyes of bilaterally injected patients experienced an average gain from nadir1 in best-corrected visual acuity (BCVA) equal to +23 ETDRS letters, greater than the 18-letter average gain among patients with only one eye injected. In both groups, the clinical improvement exceeded the conventional definition of clinical meaningfulness, in which a 15-letter gain is considered meaningful. The safety profile of the gene therapy continues to be favorable and comparable between bilaterally treated patients and unilaterally treated patients.
The results were presented at the 2024 annual meeting of the North American Neuro-Ophthalmology Society (NANOS) and will also be presented at other major medical conferences in Europe and the US later this year. Dr. Chiara La Morgia, MD, PhD, of the IRCCS Institute of Neurological Sciences of Bologna and the University of Bologna, Italy, presented the results at NANOS and commented, “It is very reassuring to clinicians that as successive waves of data become available, we continue to obtain a consistent picture of significant visual improvement among most patients treated with lenadogene nolparvovec. The evidence is becoming more robust, which is relevant for patients who are struck by a severe blinding disease such as LHON.”
These latest analyses are able to draw upon a more robust sample than the early view of the data released in March 20232. In particular, the improvements at one year post-treatment and beyond provide a more definitive view of the upward trajectory of mean visual acuity (see Figure 1).
One year after treatment, responder analyses show that the visual improvements accrue to the majority of patients: 63.2% of the eyes of bilaterally injected patients reached a clinically meaningful level of improvement in BCVA (≥0.3 LogMAR or +15 ETDRS letters), as did 57.9% of eyes of unilaterally injected patients. Nine months after injection, the average visual acuity corresponded to on-chart vision, compared to off-chart average visual acuity at baseline.
The safety results obtained in the EAPs were consistent with those observed in the clinical studies, showing a favorable safety profile of lenadogene nolparvovec. Notably, intraocular inflammation events reported in LUMEVOQ®-treated eyes were comparable in frequency, intensity, and location to those observed in the clinical studies.
“Witnessing results like these energizes the GenSight team’s drive to restore early access availability for eligible patients by Q3 this year,” said Laurence Rodriguez, Chief Executive Officer of GenSight. “As the evidence accumulates that access to LUMEVOQ helps LHON patients, in clinical trials and in real-life settings, we are working on all options to bring LUMEVOQ to patients as quickly as possible.”
LUMEVOQ® (GS010; lenadogene nolparvovec) was made available through EAPs in selected countries based on unsolicited requests from clinicians and where authorized for early access use by local regulations in those countries. The Company aims to resume early access in France under the Autorisation de l’accès compassionnel (AAC) program in Q3 2024.
About GenSight Biologics
GenSight Biologics S.A. is a clinical-stage biopharma company focused on discovering and developing innovative gene therapies for retinal neurodegenerative diseases and central nervous system disorders. GenSight Biologics’ pipeline leverages two core technology platforms, the Mitochondrial Targeting Sequence (MTS) and optogenetics, to help preserve or restore vision in patients suffering from blinding retinal diseases. Using its gene therapy-based approach, GenSight Biologics’ product candidates are designed to be administered in a single treatment to each eye by intravitreal injection to offer patients a sustainable functional visual recovery.
About Leber Hereditary Optic Neuropathy (LHON)
Leber Hereditary Optic Neuropathy (LHON) is a rare maternally inherited mitochondrial genetic disease, characterized by the degeneration of retinal ganglion cells that results in brutal and irreversible vision loss that can lead to legal blindness, and mainly affects adolescents and young adults. LHON is associated with painless, sudden loss of central vision in the 1st eye, with the 2nd eye sequentially impaired. It is a symmetric disease with poor functional visual recovery. 97% of subjects have bilateral involvement at less than one year of onset of vision loss, and in 25% of cases, vision loss occurs in both eyes simultaneously.
About LUMEVOQ® (GS010; lenadogene nolparvovec)
LUMEVOQ® (GS010; lenadogene nolparvovec) targets Leber Hereditary Optic Neuropathy (LHON) by leveraging a mitochondrial targeting sequence (MTS) proprietary technology platform, arising from research conducted at the Institut de la Vision in Paris, which, when associated with the gene of interest, allows the platform to specifically address defects inside the mitochondria using an AAV vector (Adeno-Associated Virus). The gene of interest is transferred into the cell to be expressed and produces the functional protein, which will then be shuttled to the mitochondria through specific nucleotidic sequences in order to restore the missing or deficient mitochondrial function. “LUMEVOQ” was accepted as the invented name for GS010 (lenadogene nolparvovec) by the European Medicines Agency (EMA) in October 2018. LUMEVOQ® (GS010; lenadogene nolparvovec) has not been registered in any country at this stage.
| _________________________________ |
| 1 Nadir = lowest observed visual acuity between baseline (time of treatment) and the time point of interest. |
| 2 “GenSight Biologics Announces Presentation of LUMEVOQ® Efficacy and Safety Data from Early Access Programs for ND4-LHON Patients at NANOS 2023”, press release, March 15, 2023 |
View source version on businesswire.com: https://www.businesswire.com/news/home/20240305810568/en/
Contacts
GenSight Biologics
Chief Financial Officer
Ivan Tortet
itortet@GENSIGHT-BIOLOGICS.com
LifeSci Advisors
Investor Relations
Guillaume van Renterghem
gvanrenterghem@lifesciadvisors.com
+41 (0)76 735 01 31
Source: GenSight Biologics S.A.
(Graphic: Business Wire)
View this news release and multimedia online at:
http://www.businesswire.com/news/home/20240305810568/en







