GenSight Biologics reported initial efficacy and safety results at 4 yearsa post-treatment administration in the REFLECT Phase III clinical trial with LUMEVOQ®.
- Sustained efficacy 4 years after injection in REFLECT Phase III trial
- Continued benefit at 4 years in bilaterally treated patients: clinically meaningful improvement of at least 15 letters relative to their observed nadir
- 73% of bilaterally treated patients experience clinically meaningful level of improvement
- Favorable safety profile confirmed at 4 years
PARIS--(BUSINESS WIRE)-- Regulatory News:
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20240319984906/en/
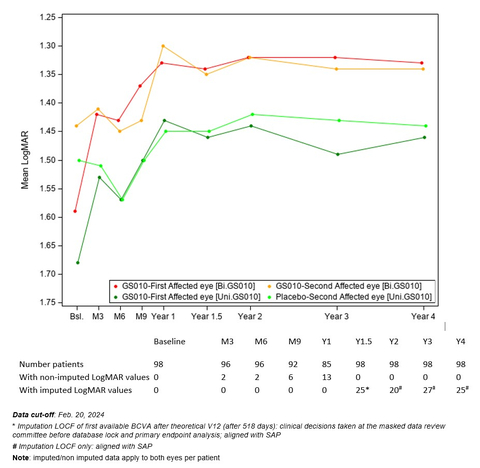
Graph 1: Evolution of Best-Corrected Visual Acuity (BCVA) Over Time – REFLECT Phase III Study (Graphic: Business Wire)
GenSight Biologics (Euronext: SIGHT, ISIN: FR0013183985, PEA-PME eligible), a biopharma company focused on developing and commercializing innovative gene therapies for retinal neurodegenerative diseases and central nervous system disorders, today reported initial efficacy and safety results at 4 yearsa post-treatment administration in the REFLECT Phase III clinical trial with LUMEVOQ® (GS010; lenadogene nolparvovec). The results show that four years after a one-time administration of the gene therapy, the visual acuity improvement has been sustained while maintaining a favorable safety profile. Bilateral injection provides an additional effect compared to unilateral treatment, demonstrated across all analyses of visual acuity improvement and responder rates.
“The latest REFLECT data confirms that the improvement seen with lenadogene nolparvovec is sustained 4 years after treatment has been given, including the additional benefit observed in participants receiving a bilateral intravitreal injection of the gene therapy,” said Patrick Yu-Wai-Man, MD, PhD, Professor of Ophthalmology, University of Cambridge and Moorfields Eye Hospital, and International Principal Investigator of REFLECT. “Importantly, REFLECT participants receiving a bilateral injection had a comparable safety profile to those treated unilaterally.”
The findings reinforce the results observed at 3 years post-treatment administration, which were reported in March 2023.b
Sustained and meaningful efficacy at Year 4
The evolution of the visual acuity over time shows that visual improvement after lenadogene nolparvovec treatment was maintained over 4 years in all subjects, with the visual acuity of bilateral injected patients remaining better than that of patients who received a unilateral injection. This difference has been observed since Year 1.5.
Graph 1: Evolution of Best-Corrected Visual Acuity (BCVA) Over Time – REFLECT Phase III Study
(See Graph 1)
Compared against nadir (i.e., the worst BCVA recorded from baseline to Year 4), average visual acuity for all LUMEVOQ-treated eyes increased beyond the +15 letter threshold that conventionally defines clinically meaningful improvement. The improvement of placebo eyes highlights the consistent contralateral treatment effect observed in all clinical trials (also documented in sham-treated eyes in the REVERSE1 and RESCUE2 trials).
“The sustained effect on vision, as observed in the REFLECT trial, is a crucial piece of the LUMEVOQ story for patients, physicians and health authorities,” commented Laurence Rodriguez, Chief Executive Officer of GenSight Biologics. “The durable impact from a single administration differentiates gene therapy from other treatment modalities, facilitating patient adherence and improving quality of life.”
Table 1: Change in Best-Corrected Visual Acuity (BCVA) versus Nadir 4 Years after Injection
|
4-year |
||
|
1st affected eye |
2nd affected eye |
|
|
Subjects bilaterally injected with LUMEVOQ® |
LUMEVOQ® |
LUMEVOQ® |
|
Subjects unilaterally injected with LUMEVOQ® |
LUMEVOQ® |
PLACEBO |
|
Data cut-off: Feb 20, 2024. Subjects bilaterally treated: 1st affected eyes: n=48; 2nd affected eyes: n=48; subjects unilaterally treated: 1st affected eyes: n=50; 2nd affected eyes: n=50. p<0.0001 for all eye groups using linear mixed model. |
||
Responder analyses reinforce the finding of improved outcomes for patients, for whom natural history typically results in greatly impaired vision with a very low likelihood of spontaneous recovery.3 Four years after a bilateral injection, 73% of patients had experienced a clinically meaningful improvement of at least -0.3 LogMAR (+15 ETDRS letters) relative to their observed nadir. 81% of bilaterally treated patients are able to read letters on a screen (on-chart vision), with the likelihood of reaching this level of vision being twice as high with bilateral treatment versus a unilateral injection (odds ratio: 2.0 [0.7; 5.5]).
Favorable safety profile
The favorable safety profile of LUMEVOQ® continued to be confirmed, with the safety profile of the drug being demonstrated as comparable in bilaterally and unilaterally treated subjects. There was no study discontinuation related to systemic or ocular adverse events, and there were no serious ocular adverse events. The main ocular adverse event was intraocular inflammation, which was mostly mild and responsive to conventional treatment.
REFLECT is a randomized, double-masked, placebo-controlled Phase III trial involving 98 subjects with vision loss due to Leber Hereditary Optic Neuropathy (LHON) caused by a mutated ND4 mitochondrial gene; enrolled ND4 subjects had vision loss up to one year from onset. All subjects received an intravitreal injection (IVT) of lenadogene nolparvovec in their first affected eye. The second affected eye was randomized to either a second IVT of LUMEVOQ® or a placebo IVT, which was administered on the same day or the following day. 48 subjects were randomized to LUMEVOQ® bilateral treatment, and 50 to lenadogene nolparvovec unilateral treatment (first-affected eye treated with LUMEVOQ®, second-affected eye treated with placebo). REFLECT patients will be followed up to 5 years post-injection to monitor the efficacy and safety of LUMEVOQ® over time.
|
References: |
|
About GenSight Biologics
GenSight Biologics S.A. is a clinical-stage biopharma company focused on discovering and developing innovative gene therapies for retinal neurodegenerative diseases and central nervous system disorders. GenSight Biologics’ pipeline leverages two core technology platforms, the Mitochondrial Targeting Sequence (MTS) and optogenetics, to help preserve or restore vision in patients suffering from blinding retinal diseases. GenSight Biologics’ lead product candidate, GS010, is in Phase III trials in Leber Hereditary Optic Neuropathy (LHON), a rare mitochondrial disease that leads to irreversible blindness in teens and young adults. Using its gene therapy-based approach, GenSight Biologics’ product candidates are designed to be administered in a single treatment to each eye by intravitreal injection to offer patients a sustainable functional visual recovery.
About Leber Hereditary Optic Neuropathy (LHON)
Leber Hereditary Optic Neuropathy (LHON) is a rare maternally inherited mitochondrial genetic disease, characterized by the degeneration of retinal ganglion cells that results in brutal and irreversible vision loss that can lead to legal blindness, and mainly affects adolescents and young adults. LHON is associated with painless, sudden loss of central vision in the 1st eye, with the 2nd eye sequentially impaired. It is a symmetric disease with poor functional visual recovery. 97% of subjects have bilateral involvement at less than one year of onset of vision loss, and in 25% of cases, vision loss occurs in both eyes simultaneously.
About LUMEVOQ® (GS010; lenadogene nolparvovec)
LUMEVOQ® (GS010; lenadogene nolparvovec) targets Leber Hereditary Optic Neuropathy (LHON) by leveraging a mitochondrial targeting sequence (MTS) proprietary technology platform, arising from research conducted at the Institut de la Vision in Paris, which, when associated with the gene of interest, allows the platform to specifically address defects inside the mitochondria using an AAV vector (Adeno-Associated Virus). The gene of interest is transferred into the cell to be expressed and produces the functional protein, which will then be shuttled to the mitochondria through specific nucleotidic sequences in order to restore the missing or deficient mitochondrial function. “LUMEVOQ” was accepted as the invented name for GS010 (lenadogene nolparvovec) by the European Medicines Agency (EMA) in October 2018. LUMEVOQ® (GS010; lenadogene nolparvovec) has not been registered in any country at this stage.
About REFLECT
REFLECT is a multi-center, randomized, double-masked, placebo-controlled study to evaluate the safety and efficacy of bilateral injections of GS010 in subjects with LHON due to the NADH dehydrogenase 4 (ND4) mutation. In the active arm, GS010 was administered as a single intravitreal injection in each eye of each subject. In the placebo arm, GS010 was administered as a single intravitreal injection to the first affected eye, while the fellow eye received a placebo injection.
The primary endpoint for the REFLECT trial is the BCVA reported in LogMAR at 1.5 years (78 weeks) post-treatment in the second‑affected/not‑yet‑affected eye. The change from baseline in second‑affected/not‑yet‑affected eyes receiving GS010 and placebo is the primary response of interest. The secondary efficacy endpoints include: change from baseline in BCVA reported in LogMAR at 2, 3, 4 and 5 years post-treatment in the second‑affected/not‑yet‑affected eye compared to both placebo and the first‑affected eye receiving GS010, change from baseline in OCT and contrast sensitivity as well as quality of life scales.
The trial was conducted in multiple centers across Europe/UK (1 each in France, Spain, Italy and the UK), the US (6 centers) and Taiwan (1 center). The trial planned to enroll 90 subjects with vision loss up to 1 year in duration; 98 subjects were successfully screened and treated. The first subject was treated in March 2018 and the last one in July 2019. Long-term follow-up is ongoing.
ClinicalTrials.gov Identifiers:
REFLECT: NCT03293524
|
a Data cut-off: February 20, 2024 |
|
b “GenSight Biologics Confirms Sustained Efficacy and Safety of Bilateral LUMEVOQ® Injections at 3-Year Follow-Up of REFLECT Phase III Trial”, press release, March 13, 2023 |
View source version on businesswire.com: https://www.businesswire.com/news/home/20240319984906/en/
Contacts
GenSight Biologics
Chief Financial Officer
Ivan Tortet
itortet@GENSIGHT-BIOLOGICS.com
LifeSci Advisors
Investor Relations
Guillaume van Renterghem
gvanrenterghem@lifesciadvisors.com
+41 (0)76 735 01 31
Source: GenSight Biologics
Graph 1: Evolution of Best-Corrected Visual Acuity (BCVA) Over Time – REFLECT Phase III Study (Graphic: Business Wire)
View this news release and multimedia online at:
http://www.businesswire.com/news/home/20240319984906/en







