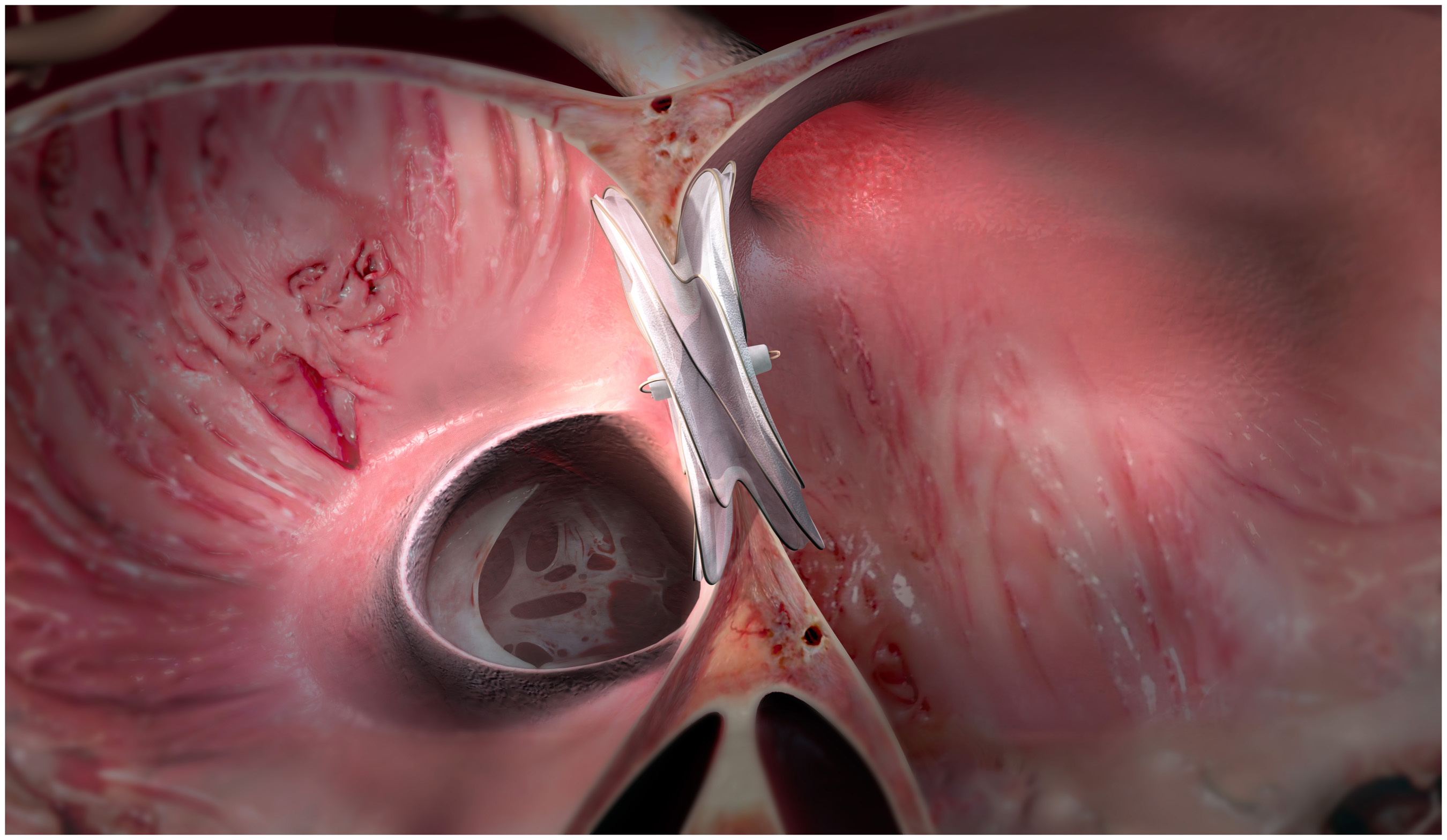
Data from the Gore ASSURED Clinical Study demonstrated 100 percent closure success at six months
| FLAGSTAFF, Ariz., June 4, 2019 /PRNewswire/ -- W. L. Gore & Associates, Inc. (Gore) announced the U.S. Food and Drug Administration’s (FDA’s) premarket approval (PMA) of the GORE® CARDIOFORM ASD Occluder for the percutaneous closure of ostium secundum atrial septal defects (ASDs). The FDA approval was supported by data collected from the pivotal stage of the Gore ASSURED Clinical Study which demonstrated 100 percent closure success at the six month evaluation in patients with a successful implant. Experience the interactive Multichannel News Release here: https://www.multivu.com/players/English/8553851-gore-cardioform-asd-occluder-receives-fda-approval/ The pivotal study evaluated the safety and efficacy of ASD closure using the GORE CARDIOFORM ASD Occluder in 125 patients with evidence of right heart volume overload demonstrating the need for defect closure. The study involved patients between the ages of 2 and 84, across 22 investigation sites, including 15 children’s hospitals. The pivotal study met its safety, closure, and technical success primary endpoints. “The FDA approval of the GORE CARDIOFORM ASD Occluder is a significant milestone for innovation in the minimally invasive treatment of ASDs,” said Matthew J. Gillespie, M.D., Children’s Hospital of Philadelphia, co-principal investigator of the ASSURED Study. “This soft, conformable device was not previously available for this range of defects but is now an option for larger defects that typically have a greater risk for complications, including right heart enlargement, atrial fibrillation, and pulmonary hypertension. The ability to retrieve and reposition the GORE CARDIOFORM ASD Occluder helps me ensure proper positioning and offers me confident closure.” The GORE CARDIOFORM ASD Occluder’s anatomically adaptable waist conforms to the defect to close ASDs from 8 mm to 35 mm in diameter, including those without a retro-aortic rim, by facilitating optimal tissue ingrowth, while maintaining thromboresistance. As the latest extension to the GORE CARDIOFORM Occluder family, it builds on a legacy of safety outcomes. “We developed the GORE CARDIOFORM ASD Occluder in partnership with leading interventional cardiologists around the globe, and its design is informed by decades of experience in technological innovation and dedication to improving patient care,” said Jake Goble, M.B.A., Ph.D., Gore Structural Heart Pipeline Leader. “This new addition extends what physicians can achieve with the GORE CARDIOFORM Occluder family.” In addition to the GORE CARDIOFORM ASD Occluder, the occluder portfolio also includes the GORE® CARDIOFORM Septal Occluder which is indicated for ASD closure for defects up to 17 mm and received U.S. Food and Drug Administration approval in 2018 for patent foramen ovale (PFO) closure to prevent recurrent ischemic stroke. The FDA approval for the GORE CARDIOFORM Septal Occluder was supported by positive results announced in May 2018 from the Gore REDUCE Clinical Study, which demonstrated the safety and efficacy of PFO closure with a Gore device plus antiplatelet therapy compared to antiplatelet therapy alone in patients with a PFO and history of cryptogenic stroke. *For complete indications and other important safety information for Gore commercial products referenced herein, refer to the applicable Instructions for Use (IFU). MEDICAL PRODUCTS DIVISIONS ABOUT GORE Contact Information Products listed may not be available in all markets. GORE®, GORE-TEX®, and CARDIOFORM are trademarks of W. L. Gore & Associates 


SOURCE W. L. Gore & Associates |




