Halberd Corporation (OTC PINK:HALB) has made a medical breakthrough in the treatment of neurodegenerative diseases.
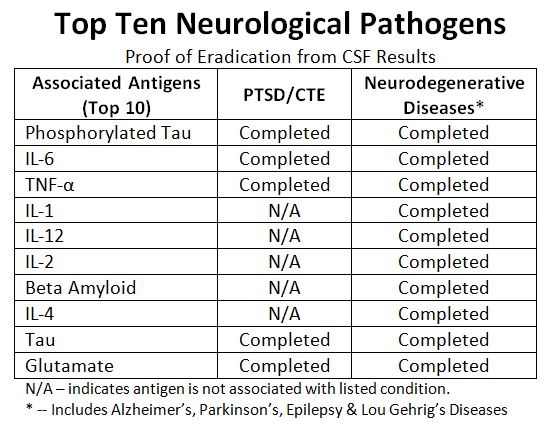
JACKSON CENTER, PA / ACCESSWIRE / February 22, 2022 / Halberd Corporation (OTC PINK:HALB) has made a medical breakthrough in the treatment of neurodegenerative diseases. With the successful elimination of glutamate from cerebral spinal fluid (CSF), Halberd has demonstrated the capability to control each of the top ten neurodegenerative disease-associated antigens. See Table Below. This could lead to an entirely new way of treating PTSD, Traumatic Brain Injury (TBI), Chronic Traumatic Encephalopathy (CTE), Alzheimer's Disease, Parkinson's Disease, Epilepsy and other neurodegenerative diseases. Halberd's unique approach allows for precise control of the level of inflammatory cytokines, proteins, and amino acids in CSF to produce and maintain healthy brain function.
Dr. Mitchell S. Felder, Halberd's Chief Technology Officer and a board-certified attending neurologist stated, "Glutamate is associated with a number of neurodegenerative conditions. Being able to precisely control glutamate levels in a patient would provide a powerful tool in the treatment of these conditions."
Dr. William G. Sturrus, Chairman of the Physics, Astronomy, Geology, and Environmental Science Department at Youngstown State University, who oversees Halberd's patented laser and radio frequency test program, commented, "It is exciting to see a single approach demonstrating such overwhelming success given the large range of antigen sizes addressed. We have experienced complete elimination of all 10 neurodegenerative disease-associated antigens in the lab, and we find elimination occurs in especially short laser exposure times. It is difficult to see how this method could be overlooked as an improved way to treat neurodegenerative diseases."
William A. Hartman, Halberd's Chairman, President & CEO added, "This breakthrough will give hope to the 36 million Americans annually, and the millions more around the world, suffering from some form of neurodegenerative diseases. I am proud of the accomplishments of our team in overcoming various obstacles in eliminating all 10 of the top neurological pathogens. We said we would do it, and we did it!
"Our next initiatives are proving efficacy in blood serum and animal testing. Not missing a step, we are in discussions with a major university that specializes in veterinary medicine to undertake animal testing. We have also contacted several Clinical Research Organizations to investigate FDA certification requirements."
Hartman continued, "As an approved government contractor, we updated our already-approved white paper with the Department of Defense regarding our scientific breakthrough. We similarly informed our NFL representative and NCAA contact to seek their organization's participation in and/or endorsement of our program to develop an efficacious treatment for traumatic brain injuries and the subsequent neurodegenerative diseases that often follow."
To view informative videos on Halberd's work, see:
Dr. Felder interviewed by Alec Torelli (https://www.youtube.com/watch?v=pTM_wQRArOE)
William Hartman interviewed by YSU (https://halberdcorporation.com/wp-content/uploads/2022/02/Alumni-Spotlight-William-A.-Hartman-64.mp4)
To get the latest news on Halberd's exciting developments, including our ongoing disease eradication accomplishments, subscribe by submitting this form.
(https://halberdcorporation.com/contact-us/)
For more information please contact:
William A. Hartman
w.hartman@halberdcorporation.com
support@halberdcorporation.com
www.halberdcorporation.com
Twitter: @HalberdC
About Halberd Corporation.
Halberd Corporation (OTC PINK:HALB), is a publicly traded company on the OTC Market, and is in full compliance with OTC Market reporting requirements. Since its restructuring in April of 2020, Halberd has obtained exclusive worldwide rights to three issued patents and has filed twenty related provisional, PCT, or utility patent applications to enhance its value to its stockholders and to attract the interests of potential development partners.
Safe Harbor Notice
Certain statements contained herein are "forward-looking statements" (as defined in the Private Securities Litigation Reform Act of 1995). The Company cautions that statements, and assumptions made in this news release constitute forward-looking statements and makes no guarantee of future performance. Forward-looking statements are based on estimates and opinions of management at the time statements are made. These statements may address issues that involve significant risks, uncertainties, estimates made by management. Actual results could differ materially from current projections or implied results. The Company undertakes no obligation to revise these statements following the date of this news release.
SOURCE: Halberd Corporation
View source version on accesswire.com:
https://www.accesswire.com/689750/Halberd-Medical-Breakthrough-for-the-Treatment-of-Neurodegenerative-Diseases-Affecting-36-Million-Americans-Annually




