Hoth Therapeutics, Inc. today announced that it has completed its analysis of the data from its Phase 1b clinical trial of BioLexa as a therapeutic for atopic dermatitis.
|
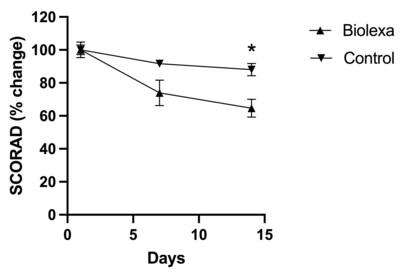
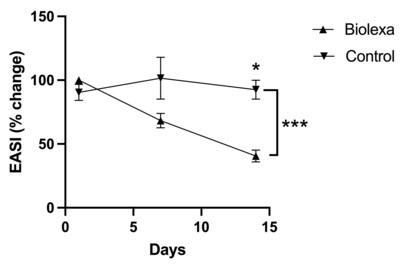
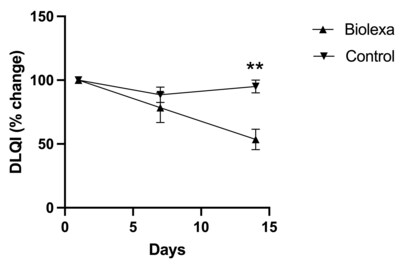
78% of patients reported that their overall health had improved since starting BioLexa as compared to 22% stating that there was no change in their health, or their health was worse. DLQI found that BioLexa use led to a 46% improvement in patient quality of life whereas placebo had no effect. NEW YORK, Sept. 6, 2023 /PRNewswire/ --Hoth Therapeutics Inc. (NASDAQ: HOTH), a patient-focused biopharmaceutical company, today announced that it has completed its analysis of the data from its Phase 1b clinical trial of BioLexa as a therapeutic for atopic dermatitis. Patients were randomized into 3 blinded treatments of the BioLexa lotion, gentamicin lotion, or placebo over a 14-day treatment period. Patients eligible to be enrolled in this trial had mild to moderate disease. Treatment with BioLexa over the 14-day treatment period led to a 35% improvement in SCORAD ("SCORing Atopic Dermatitis") measurement and a 60% improvement in EASI (Eczema Area and Severity Index) measurement of atopic skin disease, whereas gentamicin lotion and placebo had no effect. In addition, patient-reported DLQI (Dermatology Life Quality Index questionnaire) found that BioLexa use led to a 46% improvement in patient quality of life, whereas gentamicin lotion and placebo had no effect. Finally, when asked about their general impression of care since starting the study, 78% of patients reported that their overall health had improved since starting BioLexa, as compared to 22% stating that there was no change in their health, or their health was worse. Of the 78% of patients that reported an improvement in health, 42% of them said that their health was "much improved" or "very much improved". We feel these early data results strongly support further investigation of BioLexa as a steroid sparing therapeutic for atopic dermatitis. BioLexa is a patented, proprietary antimicrobial topical formulation being developed for treatment of diseases mediated by Staphylococcal biofilms. Bacterial biofilms are specialized communities consisting of bacteria adhered to a surface (both biological and abiotic surfaces) and to other bacteria, and often with a protective extracellular matrix. Mature bacterial biofilms often result in chronic, recurrent infections that are difficult to treat due to the barrier effect of the biofilm that facilitates antibiotic resistance and avoiding immune system mechanisms. The BioLexa formulation is optimized to prevent Staphylococcal biofilm formation, keeping the bacteria in a more susceptible state to antimicrobial therapy. This novel mechanism of action has the potential to broadly treat clinical manifestations resulting from Staphylococcal biofilm formation. About Hoth Therapeutics, Inc. Hoth Therapeutics is a clinical-stage biopharmaceutical company dedicated to develop innovative, impactful, and ground-breaking treatments with a goal to improve patient quality of life. We are a catalyst in early-stage pharmaceutical research and development, elevating drugs from the bench to pre-clinical and clinical testing. Utilizing a patient-centric approach, we collaborate and partner with a team of scientists, clinicians, and key opinion leaders to seek out and investigate therapeutics that hold immense potential to create breakthroughs and diversify treatment options. To learn more, please visithttps://ir.hoththerapeutics.com/ . Forward-Looking Statement This press release includes forward-looking statements based upon Hoth's current expectations which may constitute forward-looking statements for the purposes of the safe harbor provisions under the Private Securities Litigation Reform Act of 1995 and other federal securities laws, and are subject to substantial risks, uncertainties and assumptions. These statements concern Hoth's business strategies; the timing of regulatory submissions; the ability to obtain and maintain regulatory approval of existing product candidates and any other product candidates we may develop, and the labeling under any approval we may obtain; the timing and costs of clinical trials, the timing and costs of other expenses; market acceptance of our products; the ultimate impact of the current Coronavirus pandemic, or any other health epidemic, on our business, our clinical trials, our research programs, healthcare systems or the global economy as a whole; our intellectual property; our reliance on third party organizations; our competitive position; our industry environment; our anticipated financial and operating results, including anticipated sources of revenues; our assumptions regarding the size of the available market, benefits of our products, product pricing, timing of product launches; management's expectation with respect to future acquisitions; statements regarding our goals, intentions, plans and expectations, including the introduction of new products and markets; and our cash needs and financing plans. There are a number of factors that could cause actual events to differ materially from those indicated by such forward-looking statements. You should not place reliance on these forward-looking statements, which include words such as "could," "believe," "anticipate," "intend," "estimate," "expect," "may," "continue," "predict," "potential," "project" or similar terms, variations of such terms or the negative of those terms. Although the Company believes that the expectations reflected in the forward-looking statements are reasonable, the Company cannot guarantee such outcomes. Hoth may not realize its expectations, and its beliefs may not prove correct. Actual results may differ materially from those indicated by these forward-looking statements as a result of various important factors, including, without limitation, market conditions and the factors described in the section entitled "Risk Factors" in Hoth's most recent Annual Report on Form 10-K and Hoth's other filings made with the U. S. Securities and Exchange Commission. All such statements speak only as of the date made. Consequently, forward-looking statements should be regarded solely as Hoth's current plans, estimates, and beliefs. Investors should not place undue reliance on forward-looking statements. Hoth cannot guarantee future results, events, levels of activity, performance or achievements. Hoth does not undertake and specifically declines any obligation to update, republish, or revise any forward-looking statements to reflect new information, future events or circumstances or to reflect the occurrences of unanticipated events, except as may be required by applicable law. Investor Contact:
SOURCE Hoth Therapeutics, Inc. |
||
Company Codes: NASDAQ-NMS:HOTH |