IceCure Medical Ltd. announced the latest release of a study in a series of independent studies of ProSense® published in peer-reviewed journals demonstrating safety and efficacy.
- Independent study concluded:
- IceCure system offers significant advantage in ability to re-treat tumors that are initially resistant, achieving a subsequent local control rate of 100%
- 92.4% of patients (N= 24) were discharged the day after cyroablation
- The technology’s ability to preserve renal function post-treatment is paramount for patients’ quality of life
- Findings serve as a guide for medical professionals in choosing efficient, cost-effective, and patient-friendly treatment options, thereby benefiting society at large by optimizing kidney tumor management - Success across multiple indications supports ProSense®'s commercialization, particularly in facilities that can use one device across multiple specialties
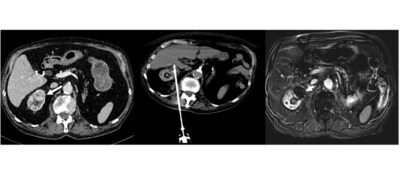
CAESAREA, Israel, Nov. 28, 2023 /PRNewswire/ -- IceCure Medical Ltd. (Nasdaq: ICCM), developer of the ProSense® System, a minimally-invasive cryoablation technology that destroys tumors by freezing as an alternative to surgical tumor removal, today announced the latest release of a study (the “Study”) in a series of independent studies of ProSense® published in peer-reviewed journals demonstrating safety and efficacy. The Study, titled “Single-Probe Percutaneous Cryoablation with Liquid Nitrogen for the Treatment of T1a Renal Tumors”, published in Cancers, demonstrated the safety and efficacy of ProSense® in treating malignant small renal masses. The Study was authored by eight physicians in France, including interventional radiologists and urologists from Curie Institute, Paris, Nîmes University Hospital (University of Montpellier), Nîmes, and Carémeau University Hospital, Nîmes.
ProSense® is approved for the treatment of benign and malignant kidney tumors in the U.S., Europe, and numerous other countries.
The objective of this retrospective Study was to address the challenges of managing small renal masses, including recurrence rates, by exploring the safety and efficiency of single-probe percutaneous cryoablation as a potential solution. The causes of partial tumor response and persistent tumor residue after a T1a renal cryoablation procedure were assessed. A total of 25 patients underwent cryoablation for 26 T1a renal tumors with a median tumor size of 25.3 mm (20 to 30.7 mm) and a median RENAL nephrometry score, indicating tumor complexity, of 7 (5 to 9).
Main findings of the Study:
- Disease-free survival rate was 92% (23 out of 25) at a median follow-up of 26 and a half months
- Recurrent lesions were treated again using cryoablation, achieving a secondary local control rate of 100%
- No patients died
- No major complications arose
- 92.4% of patients (N= 24) were discharged the day after surgery
One of the Study’s authors, Professor Julien Frandon, Director of the Interventional Radiology Department at Nîmes University Hospital commented, “In our recent publication, we evaluated the safety and efficacy of IceCure’s cryoablation technology for the treatment of renal T1a tumors. This innovative approach has demonstrated remarkable safety profiles, even for challenging and unfavorably located small renal masses. One of the study’s crucial findings is the technology’s ability to preserve renal function post-treatment, which is paramount for patients’ quality of life. This technology stands out as a forward-thinking solution in oncological treatment, providing a combination of patient safety, procedural efficiency, and cost-effectiveness.”
“We appreciate the diligent work of Professor Frandon and his colleagues in conducting this Study and we congratulate them on its publication in Cancers, a prestigious European medical journal,” stated IceCure’s Chief Executive Officer Eyal Shamir. “The authors’ findings are similar to the interim results from our own ICESECRET study in small renal masses which demonstrated an 89.5% recurrence-free rate. We expect ICESECRET’s five-year patient follow-up to be completed in 2026, with topline results available shortly afterwards.”
About ProSense®
ProSense® cryoablation is a minimally invasive, non-surgical, outpatient 40-minute, cost affective treatment option that destroys tumors by freezing them. ProSense® has been investigated and proven effective in various clinical applications, including breast tumors, kidney cancer, lung cancer, and in palliative care. Independent and company-sponsored clinical studies of ProSense® have shown strong results, with high rates of tumor destruction, and patient and physician satisfaction.
About IceCure Medical
IceCure Medical (Nasdaq: ICCM) develops and markets ProSense®, an advanced liquid-nitrogen-based cryoablation therapy for the treatment of tumors (benign and cancerous) by freezing, with the primary focus areas being breast, kidney, bone and lung cancer. Its minimally invasive technology is a safe and effective alternative to hospital surgical tumor removal that is easily performed in a relatively short procedure. The system is marketed and sold worldwide for the indications cleared and approved to date including in the U.S., Europe, and China.
Forward Looking Statements
This press release contains forward-looking statements within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995 and other Federal and Israeli securities laws. Words such as “expects,” “anticipates,” “intends,” “plans,” “believes,” “seeks,” “estimates” and similar expressions or variations of such words are intended to identify forward-looking statements. For example, IceCure is using forward looking statement in this press release when it discusses: the potential of ProSense® to be an effective and viable option for treating small renal masses; and the expectation that the five-year patient follow-up for the ICESECRET study is to be completed in 2026, with topline results available shortly afterwards. Historic results of scientific research and clinical and preclinical trials do not guarantee that the conclusions of future research or trials will suggest identical or even similar conclusions. Because such statements deal with future events and are based on IceCure’s current expectations, they are subject to various risks and uncertainties and actual results, performance, or achievements of IceCure could differ materially from those described in or implied by the statements in this press release. Important factors that could cause actual results, developments and business decisions to differ materially from those anticipated in these forward-looking statements include, among others: the Company’s planned level of revenues and capital expenditures; the Company’s available cash and its ability to obtain additional funding; the Company’s ability to market and sell its products; legal and regulatory developments in the United States and other countries; the Company’s ability to maintain its relationships with suppliers, distributors and other partners; the Company’s ability to maintain or protect the validity of its patents and other intellectual property; the Company’s ability to expose and educate medical professionals about its products; political, economic and military instability in the Middle East, specifically in Israel; as well as those factors set forth in the Risk Factors section of the Company’s Annual Report on Form 20-F for the year ended December 31, 2022 filed with the SEC on March 29, 2023, and other documents filed with or furnished to the SEC which are available on the SEC’s website, www.sec.gov. The Company undertakes no obligation to update these statements for revisions or changes after the date of this release, except as required by law.
IR Contact:
Email: investors@icecure-medical.com
Michael Polyviou
Phone: 732-232-6914
Todd Kehrli
Phone: 310-625-4462
Photo - https://mma.prnewswire.com/media/2286054/IceCure_cryoprobe.jpg
Logo - https://mma.prnewswire.com/media/1941429/IceCure_Logo.jpg
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/icecure-system-successfully-treated-kidney-cancer-tumor-with-92-disease-free-survival-rate-and-100-secondary-local-control-rate-301998960.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/icecure-system-successfully-treated-kidney-cancer-tumor-with-92-disease-free-survival-rate-and-100-secondary-local-control-rate-301998960.html
SOURCE IceCure Medical
Company Codes: NASDAQ-NMS:ICCM