The Janssen Pharmaceutical Companies announced today that five company-sponsored presentations will be featured during the Association for Research in Vision and Ophthalmology (ARVO) 2023 Annual Meeting in New Orleans from April 23-27, 2023.
|
Important new data on Janssen's investigational gene therapy portfolio, including botaretigene sparoparvovec (bota-vec) and JNJ-1887, will be presented RARITAN, N.J., April 21, 2023 /PRNewswire/ -- The Janssen Pharmaceutical Inc. of Johnson & Johnson announced today that five company-sponsored presentations will be featured during the Association for Research in Vision and Ophthalmology (ARVO) 2023 Annual Meeting in New Orleans from April 23-27, 2023. Janssen presentations will include updates from the Phase 1/2 MGT009 trial for investigational gene therapy botaretigene sparoparvovec (bota-vec, formerly AAV-RPGR) in patients with the inherited retinal disease (IRD) X-linked retinitis pigmentosa (XLRP) associated with the retinitis pigmentosa GTPase regulator (RPGR) gene, and pooled safety analysis data from two Phase 1 trials of the investigational gene therapy JNJ-81201887 (JNJ-1887); one trial in patients with geographic atrophy (GA), a late-stage and severe form of age-related macular degeneration (AMD) and one trial in wet AMD (Abstracts #5446 and #732-C0314).1,2 Experience the full interactive Multichannel News Release here: "People living with retinal diseases, including XLRP and geographic atrophy, are often faced with the inevitable fate of going blind. In this new era of innovation, we're taking a cutting-edge approach to saving sight through groundbreaking technologies such as gene therapy, and we're thrilled to showcase some of this research at this year's ARVO annual meeting," said James List, M.D., Ph.D., Global Therapeutic Area Head, whose team oversees a portfolio of programs including Retina at Janssen Research & Development, LLC. "We are on a bold mission to restore and preserve vision for people living with retinal diseases, and these important data bring us one step closer to building a brighter future for the patients we serve." XLRP is a rare condition estimated to impact one in 40,000 people globally.3,4 People with XLRP have progressive vision loss, starting in childhood with night blindness.5 Over time, they lose their peripheral vision followed by central vision loss leading to legal blindness by the average age of 40.5 Currently, no treatments are approved for XLRP.5 Geographic atrophy is an advanced form of AMD.6 It affects more than five million individuals worldwide and is a leading cause of blindness in people over 65 years of age.6 It has a devastating impact on these patients and their quality of life, including their ability to read, drive and perform other day-to-day activities.6 A complete listing of Janssen-sponsored abstracts being featured at the ARVO Annual Meeting is provided below. Abstracts can also be found on the ARVO Annual Meeting website.
Janssen will also have an interactive Retina Medical Affairs exhibit booth (location #709) at the ARVO annual meeting. For more information about Janssen Retina's research and portfolio, please visit www.retina.janssen.com. About botaretigene sparoparvovec (bota-vec) About the Janssen and MeiraGTx Strategic Collaboration About JNJ-1887 About the Janssen Pharmaceutical Companies of Johnson & Johnson Learn more at www.janssen.com. Follow us at @JanssenGlobal. Janssen Research & Development, LLC is part of the Janssen Pharmaceutical Companies of Johnson & Johnson. Cautions Concerning Forward-Looking Statements This press release contains "forward-looking statements" as defined in the Private Securities Litigation Reform Act of 1995 regarding botaretigene sparoparvovec and JNJ-81201887. The reader is cautioned not to rely on these forward-looking statements. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or known or unknown risks or uncertainties materialize, actual results could vary materially from the expectations and projections of Janssen Research & Development, LLC, any of the other Janssen Pharmaceutical Companies, and/or Johnson & Johnson. Risks and uncertainties include, but are not limited to: challenges and uncertainties inherent in product research and development, including the uncertainty of clinical success and of obtaining regulatory approvals; uncertainty of commercial success; manufacturing difficulties and delays; competition, including technological advances, new products and patents attained by competitors; challenges to patents; product efficacy or safety concerns resulting in product recalls or regulatory action; changes in behavior and spending patterns of purchasers of health care products and services; changes to applicable laws and regulations, including global health care reforms; and trends toward health care cost containment. A further list and descriptions of these risks, uncertainties and other factors can be found in Johnson & Johnson's Annual Report on Form 10-K for the fiscal year ended January 1, 2023, including in the sections captioned "Cautionary Note Regarding Forward-Looking Statements" and "Item 1A. Risk Factors," and in Johnson & Johnson's subsequent Quarterly Reports on Form 10-Q and other filings with the Securities and Exchange Commission. Copies of these filings are available online at www.sec.gov, www.jnj.com or on request from Johnson & Johnson. None of the Janssen Pharmaceutical Companies nor Johnson & Johnson undertakes to update any forward-looking statement as a result of new information or future events or developments. Media Contact: Investor Contact: 1 Caratzas, E et al. Humoral immune response to AAV5-RPGR (botaretigene sparoparvovec) gene therapy in RPGR-associated X-linked retinitis pigmentosa. Abstract #5446. Association for Research in Vision and Ophthalmology 2023 Annual Meeting. 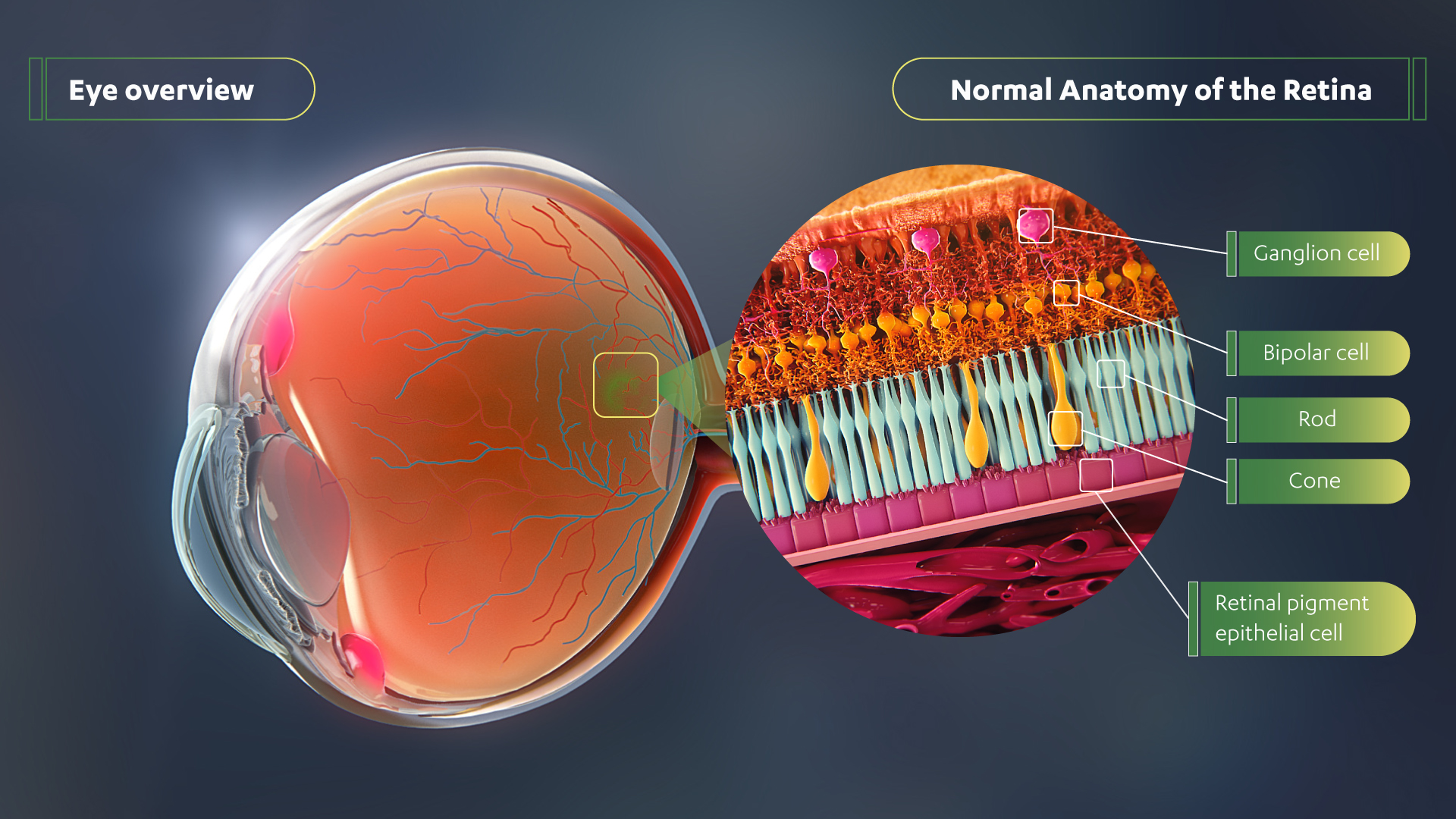
SOURCE Janssen Pharmaceutical Companies of Johnson & Johnson |




