KARL STORZ Endoscopy-America, Inc., a leader in endoscopy, imaging, and operating room integration solutions, is pleased to announce the recent publication of results from a prospective, randomized multicenter trial to evaluate use of near-infrared fluorescence cholangiography (NIFC) to detect extrahepatic biliary structures.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20191001005701/en/
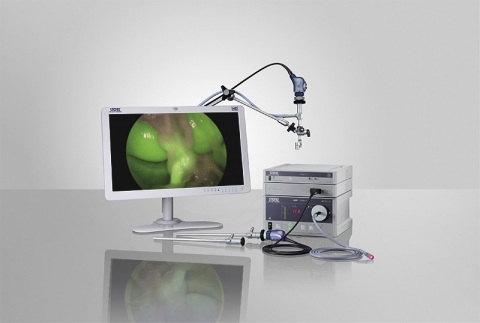
The IMAGE1 S NIR/ICG fluorescence imaging system from KARL STORZ. (Photo: Business Wire)
The trial involved four continents at seven centers in the U.S., Germany, Italy, Argentina, and Japan. All procedures were performed using the NIR/ICG Imaging System, an innovative extension of the IMAGE1 S™ adaptable video architecture (KARL STORZ SE & Co. KG, Tuttlingen, Germany). The NIR/ICG Imaging System enables surgeons to perform minimally invasive surgery using conventional endoscopic white light or using NIR/ICG fluorescence imaging for enhanced visual assessment of vessels, blood flow, and related tissue perfusion.
Use of the NIR/ICG imaging technology in combination with ICG enables enhanced visualization and evaluation of organs, associated tissues, and the bile duct. It also allows assessment of perfusion and perfusion defects. The system achieves this by extending the spectrum of available diagnostic options and assisting surgeons in making critical decisions that may also reduce the occurrence of costly complications. In essence, it enables them to more easily detect and identify suspect tissue they couldn’t see before—which provides better outcomes for the patient.
While laparoscopic cholecystectomy has become a relatively common procedure, surgeons face certain difficulties in identifying the biliary tract anatomy, which has one of the most variable anatomies in the human body. Accurate identification and prevention of potential injury to the cystic duct and common bile duct are primary challenges to surgeons performing these procedures.
“We still experience complications when performing laparoscopic cholecystectomy,” said Raul J. Rosenthal, MD, one of the authors of the recent multicenter study. “Since the introduction of laparoscopy in 1989, the incidence of common bile duct injuries has doubled from 0.4% to close to 0.8% of those undergoing this approach,” he explained. “Bile duct injuries, despite being rare, can have life‐threatening connotations, and in 97% of the cases they are due to a visual misperception: the surgeon believes that he or she is working on the cystic duct, but in reality is working on the common bile duct.”
In describing the findings of the recent trial, Rosenthal noted that, “The results are staggering, because not only were the surgeons that utilized both white light and near-infrared light able to visualize the biliary tract anatomy two to three times better than those who utilized white light alone, but also the group that utilized only white light had two common bile duct injuries in 300 cases, while the group that used both white and the near-infrared with fluorescent imaging light had no injuries.”
For more information, feel free to download copy of this important study at: https://journals.lww.com/annalsofsurgery/Abstract/publishahead/Randomized_Trial_of_Near_infrared_Incisionless.95270.aspx
About the IMAGE1 S™ System
The IMAGE1 S™ system is a single adaptable video architecture designed for multi-disciplinary and technology use. The system’s modular CCU design enables customers to acquire individual IMAGE1 S™ technologies if and when they need it. Its adaptability and scalability to match the customer’s individual needs result in the system offering the lowest total cost of ownership. The NIR/ICG capabilities within IMAGE1 S™ offer the unique opportunity to visualize perfusion in either green or blue. KARL STORZ offers a 10 mm and 5 mm solution for the visual assessment of vessels, blood flow, and related tissue perfusion for at least one of the major extrahepatic bile ducts (cystic duct, common bile duct, and common hepatic duct); a 4 mm solution for minimally invasive cranial neurosurgery; and the VITOM II ICG for tissue perfusion—all within a single architecture.
About KARL STORZ
KARL STORZ Endoscopy-America, Inc., is an affiliate of KARL STORZ SE & Co. KG, an international leader for nearly 75 years in reusable endoscope technology, encompassing all endoscopic specialties. Based in Tuttlingen, Germany, KARL STORZ SE & Co. KG is a family-owned company that designs, engineers, manufactures, and markets all its products with an emphasis on visionary design, precision craftsmanship, and clinical effectiveness. For more information, call 800-421-0837 or visit www.karlstorz.com.
View source version on businesswire.com: https://www.businesswire.com/news/home/20191001005701/en/
Contacts
Susan Jaffy Marx
Marketing Communications
KARL STORZ Endoscopy-America, Inc.
(424) 218-8701
Source: KARL STORZ Endoscopy-America, Inc.