Data published in Science Translational Medicine with Emulate’s Liver-Chip demonstrate robust reproducibility and diverse phenotypes of human liver toxicities and species-specific drug responses
Data published in Science Translational Medicine with Emulate’s Liver-Chip demonstrate robust reproducibility and diverse phenotypes of human liver toxicities and species-specific drug responses
BOSTON--(BUSINESS WIRE)-- Emulate, Inc. today announced the publication of research demonstrating that its Liver-Chip accurately modeled and predicted human toxicity in a range of eight previously-studied drugs. Results from the Liver-Chip study showed the ability to model the mechanism of action for hepatotoxicity, measure relevant clinical biomarkers, and predict species differences between human, dog, and rat liver drug toxicity for the drugs tested.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20191106005813/en/
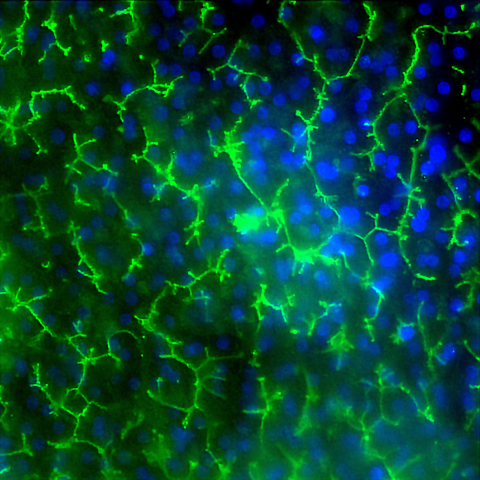
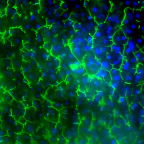
Image of Emulate’s human Liver-Chip showing multidrug resistance-associated protein 2 (MRP2 in green and DAPI in blue) efflux transporter in hepatocytes after 14 days in culture. Credit: Emulate, Inc.
The Liver-Chip data, published today in Science Translational Medicine, are co-authored by scientists from AstraZeneca, Janssen Research & Development, LLC, the Wyss Institute for Biologically Inspired Engineering at Harvard University, and Emulate. The collaborative research supports efforts to better predict drug safety and human response in the drug development process, as well as, initiatives for 3Rs programs for the reduction, refinement, and replacement of animal testing. The liver is among the organ systems most commonly affected by adverse drug reactions, and drug-induced liver injury is one of most frequent reasons for the withdrawal of drugs from the market.1
“Organs-on-Chips technology has the potential to enhance and accelerate our ability to translate science into innovative medicines for patients,” said Stefan Platz, Senior Vice President, Clinical Pharmacology and Safety Sciences, R&D, AstraZeneca. “Our collaborative work on the development and characterisation of the Liver-Chip represents valuable progress to enable us to more broadly apply this model as we aim to improve our ability to better predict adverse drug reactions before drug candidates enter clinical trials.”
The published research tested drugs for which human toxicity was not predicted by animal studies and, as a result, the drugs advanced to human clinical trials that were subsequently halted due to lack of safety. The Liver-Chip predicted the human toxicity that was missed in the animal studies.
Liver-Chip Results: Drugs Halted in Previous Clinical Trials Based on Animal Studies
|
Drugs |
Clinical Observation |
Potential mechanism of |
Liver-Chip Results |
|
Fialuridine, an anti-viral nucleoside analog |
Discontinued in Phase II clinical trial, due to liver failure and deaths in 5 out of 15 patients, caused by microvesicular steatosis |
Drug-induced mitochondrial injury leading to fat accumulation |
Observed significant lipid accumulation within hepatocytes in the Liver-Chip with increase release of sensitive liver injury markers, including miR122, α‑GST, and keratin 18 (K-18) |
|
A G-protein-coupled receptor 40 (GPR40) agonist. |
Discontinued in Phase III clinical trials |
Formation of reactive acyl glucuronide metabolites, suppression of mitochondrial respiration, and inhibition of hepatic transporters |
Human Liver-Chip detected reactive metabolite formation, hepatic drug transporter inhibition, mitochondrial dysfunction, lipid accumulation, oxidative stress, release of inflammatory cytokines |
|
Drug X |
Discontinued in Phase I clinical trial |
Kupffer cell depletion |
Kupffer cell depletion was shown by Kupffer cell marker staining and decrease. This effect was also associated with decrease in interleukin 6 (IL-6) and monocyte chemoattractant protein-1 (MCP-1) in the human Liver-Chip at clinically relevant concentration |
“These results with our Liver-Chip are a prime example of how our portfolio of Organ‑Chip products have the potential to identify possible safety issues in humans earlier and more reliably in the drug discovery and development process, with the goal of enabling the design and selection of drug candidates that have a higher potential of success in human clinical trials,” said Geraldine A. Hamilton, President and Chief Scientific Officer of Emulate. “With our integrated platform of chips, instruments, and software now available for use by researchers in the pharmaceutical industry, we are taking an important step towards our mission of transforming the R&D paradigm and enabling better prediction of how humans will respond to drug candidates. The goal is that this will ultimately lead to the advancement of better and safer medicines for the benefit of patients.”
The Liver-Chip has an advanced design that incorporates relevant extracellular matrix interactions and the key cell types in the liver (hepatocytes, stellate cells, kupffer cells, and liver sinusoidal endothelial cells). In addition, the chip supports hepatocyte and liver sinusoidal endothelial cell interface, along with relevant cytoarchitecture, and physiological flow. The Liver-Chip recreates true-to-life functions of the liver by working within the automated, lab-ready Human Emulation System that provides the dynamic flow of cell culture media and the mechanical forces that recreate the microenvironment that cells experience in the human body. Now commercially-available, the Liver-Chip can perform testing across a broad range of applications, including general toxicity, mechanistic toxicity, and nutrient metabolism studies.
About Emulate, Inc.
Emulate, Inc. is a privately held company that creates living products for understanding how diseases, medicines, chemicals, and foods affect human health. Our Human Emulation System® sets a new standard for recreating true-to-life human biology and is being used to advance product innovation, design, and safety across a range of applications including drug development, agriculture, cosmetics, food, and chemical-based consumer products. Emulate continues to develop a wide range of Organ-Chips and disease models through collaborations with industry partners and internal R&D programs. Emulate is also working with clinical partners to produce Organ-Chips personalized with an individual patient’s stem cells, for applications in precision medicine and personalized health. Our founding team pioneered the Organs-on-Chips technology at the Wyss Institute for Biologically Inspired Engineering at Harvard University. Emulate holds the worldwide exclusive license from Harvard University to a robust and broad intellectual property portfolio for the Organs-on-Chips technology and related systems.
1 Lasser KE, Allen PD, Woolhandler SJ, Himmelstein DU, Wolfe SM, Bor DH. Timing of New Black Box Warnings and Withdrawals for Prescription Medications. JAMA. 2002;287(17):2215–2220. doi:10.1001/jama.287.17.2215.
View source version on businesswire.com: https://www.businesswire.com/news/home/20191106005813/en/
Contacts
Emulate:
Kathryn Morris
kathryn@theyatesnetwork.com
914-204-6412
Source: Emulate, Inc.
Smart Multimedia Gallery
Image of Emulate’s human Liver-Chip showing multidrug resistance-associated protein 2 (MRP2 in green and DAPI in blue) efflux transporter in hepatocytes after 14 days in culture. Credit: Emulate, Inc.
Representative bright-field image of human hepatocytes within Emulate’s Liver-Chip after 7 days in culture. Credit: Emulate, Inc.
View this news release and multimedia online at:
http://www.businesswire.com/news/home/20191106005813/en