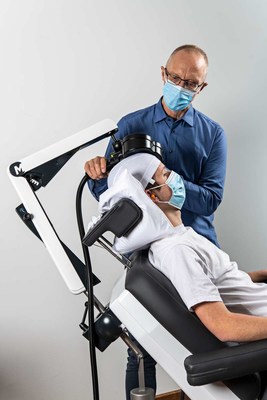
MagVenture, the Danish medical device company behind MagVenture TMS Therapy®, introduces the MagVenture Flow Arm — a patent pending*, clinical positioning system to optimize the delivery of MagVenture’s FDA cleared protocols for non-invasive brain stimulation technology within the treatment of major depressive disorder and as an adjunct therapy for OCD.
ALPHARETTA, Ga., March 17, 2021 /PRNewswire/ -- MagVenture, the Danish medical device company behind MagVenture TMS Therapy®, introduces the MagVenture Flow Arm — a patent pending*, clinical positioning system to optimize the delivery of MagVenture's FDA cleared protocols for non-invasive brain stimulation technology within the treatment of major depressive disorder and as an adjunct therapy for OCD.
"Responsiveness to customer needs is a core part of MagVenture DNA," says MagVenture SVP of Sales, Kerry Rome. "MagVenture is continuously striving to improve solutions to optimize the clinical practice of transcranial magnetic stimulation – or TMS in short. In recent years, the clinical market for TMS has expanded at such a rapid pace that manufacturers are being challenged to keep pace with the technological requirements emerging with the exponential expansion in the demand for utilization. We've seen MagVenture drive an FDA clearance for the ultra-fast 'Theta Burst' TMS which has reduced treatment times for patients from 37 to just 3 minutes. Now, with the introduction of a remarkedly smooth solution to help TMS operators quickly and easily prepare patients for treatment, MagVenture is further improving the efficiency and experience of TMS for patients and operators alike," states Kerry Rome.
The latest improvement to the MagVenture TMS Therapy® system, deemed the 'MagVenture Flow Arm', concerns the applicator 'coil'. The coil is essentially two very heavy spools of copper wire that produce and focus the magnetic fields for the non-invasive brain stimulation technique. The typically large and unwieldy coils, by necessity, have to be accurately positioned and held immobile over the brain target(s) during the TMS procedures. Over the past year, MagVenture product engineers and application experts, in close collaboration with MagVenture customers in the USA, Europe, Australia, and Asia, perfected a solution that vastly improves ease of use for MagVenture TMS operators to now, almost effortlessly, move and securely position TMS coils for non-invasive brain stimulation (TMS) procedures.
Kerry Rome goes on to add: "Throughout the R&D and testing phases for the Flow Arm project, the MagVenture organization here in the US has been waiting with much anticipation and, finally, we are super excited about the introduction of MagVenture's patent-pending 'Flow Arm' for TMS coil positioning. It's a game changer for TMS providers seeking optimal ease of use and efficiency in their clinical TMS services and, in context with the current COVID-19 pandemic, the improvements in the MagVenture reduced treatment/operator-patient interaction time is going to prove to be a big positive."
Transcranial Magnetic Stimulation is a technology that applies rapidly changing magnetic fields to stimulate specific areas in the brain. TMS has been available as a tool for non-invasive brain stimulation for more than three decades and is currently used extensively in clinical psychiatry, and for ongoing academic medical and translational neuroscience research. MagVenture TMS Therapy® is FDA cleared as a treatment for major depressive disorder (MDD) and as an adjunct therapy treatment for obsessive-compulsive disorder (OCD). MagVenture TMS Therapy® is an outpatient procedure with a well-tolerated safety profile with no systemic side effects. The most common side effects are headaches and nausea. MagVenture TMS Therapy® has been clinically available in the USA since 2015 and is currently delivered at hospitals and psychiatric practices nationwide.
Contact details: Kerry Rome, Vice President Sales, MagVenture Inc., USA: 310 213 2697, email: kr@magventure.com
MagVenture TMS Therapy® FDA clearances: "Treatment of Major Depressive Disorder in adult patients who have failed to receive satisfactory improvement from prior antidepressant medication in the current episode and as an "adjunct for the treatment of adult patients suffering from Obsessive-Compulsive Disorder (OCD)".
MagVenture is a Danish medical device company specializing in non-invasive magnetic stimulation solutions for the clinical treatments and brain research. In 2018, MagVenture was the first TMS company to receive FDA clearance for Express TMS®, reducing treatment time from the standard 37 minutes to just 3 minutes per session.
MagVenture A/S has over 25 years of experience within neuromodulation. From its headquarters in Denmark, MagVenture's products are manufactured and promoted worldwide through direct sales subsidiaries in the US, Brazil, Germany, the UK, and through an extensive global network of dedicated distributors in Europe, Asia, Middle East, and the Americas.
* Pat. EP 20214073.7 / US 63/125,594.
![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/magventure-introduces-new-solution-to-improve-the-ease-of-use-for-non-invasive-brain-stimulation-301247530.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/magventure-introduces-new-solution-to-improve-the-ease-of-use-for-non-invasive-brain-stimulation-301247530.html
SOURCE MagVenture