MediPharm Labs Corp. (TSX: LABS) (OTCQX: MEDIF) (FSE:MLZ) (“MediPharm Labs” or the “Company”) a global leader in specialized, research-driven pharmaceutical-quality cannabis extraction, distillation and derivative products, today announced that its subsidiary, MediPharm Labs Australia Pty. Ltd. (“MediPharm Labs Australia”) has entered into an agreement to supply pharmaceutical-quality (GMP certified), formulated cannabis oil products to Beacon Medical Australia Pty. Ltd.
TORONTO, June 19, 2020 (GLOBE NEWSWIRE) -- MediPharm Labs Corp. (TSX: LABS) (OTCQX: MEDIF) (FSE:MLZ) (“MediPharm Labs” or the “Company”) a global leader in specialized, research-driven pharmaceutical-quality cannabis extraction, distillation and derivative products, today announced that its subsidiary, MediPharm Labs Australia Pty. Ltd. (“MediPharm Labs Australia”) has entered into an agreement to supply pharmaceutical-quality (GMP certified), formulated cannabis oil products to Beacon Medical Australia Pty. Ltd. (“Beacon Medical Australia”), a subsidiary of VIVO Cannabis Inc. (TSX: VIVO, OTCQX: VVCIF) (“VIVO Cannabis”).
Under the agreement with Beacon Medical Australia, which has an initial one-year term subject to renewal, MediPharm Labs Australia will supply a full range of GMP-certified, formulated CBD and THC cannabis oil products to Beacon Medical Australia for further distribution under its own branding.
“Just weeks after MediPharm Labs Australia was granted GMP Certification, we’re pleased to announce this agreement that underscores the burgeoning demand for our specialized skills and rapid success in converting our opportunity pipeline into tangible, revenue-generating business,” said Warren Everitt, CEO, Asia Pacific, MediPharm Labs. “We are excited to be working with VIVO to bring high quality products to medical consumers across Australia and other emerging markets.”
“VIVO’s international efforts remain focused on the emerging Australian and German markets and we continue to seek out partnerships with organizations that have proven track records,” said Barry Fishman, CEO of VIVO. “For this reason, we are thrilled to be working with MediPharm Labs to leverage their GMP production capabilities to accelerate our Australian market presence. We are confident this will allow us to continue to provide our Australian patients with the medical-grade products they have come to expect from the Beacon Medical™ brand.”
MediPharm Labs Australia Nearing Commercialization and First Revenues
MediPharm Labs also announced that MediPharm Labs Australia has received a large shipment of 35,000 GMP-units of formulated finished products and a large volume of bulk oil for further manufacturing into finished products, from its Barrie, Ontario facility to meet its burgeoning customer needs within the Australian domestic and export cannabis markets.
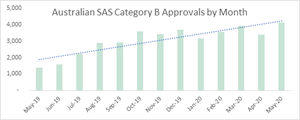
Australia remains one of the fastest growing medical cannabis markets in the world. According to the Therapeutic Goods Administration (TGA), the rate of medical cannabis approvals is accelerating in Australia. Up to May 31, 2020, the TGA had approved over 46,000 SAS Category B applications for unapproved medicinal cannabis products, with 83% of all those approvals occurring in the last 12 months alone.1 Currently, the Australian legal cannabis market is estimated at US$40 million, and as a result of various recent regulatory changes is expected to grow to US$170 million by next year, and over $1 billion by 2023.2
“Since we expect to expand our international distribution of proprietary cannabis concentrate products for sale globally, we intend to continue to use our Barrie, Ontario facility to supply finished goods to complement our asset in Australia,” said Warren Everitt, CEO, MediPharm Labs Australia. “Having two GMP-certified manufacturing platforms integrated into a global supply-chain gives us flexibility to respond to global demand in an efficient and effective manner for our customers.”
“By leveraging our deep scientific, production and regulatory expertise, we have created two strong GMP certified manufacturing platforms, secured all licenses necessary to operate on a multi-jurisdictional basis and developed a very attractive portfolio of international supply agreements that will make great use of our industry-leading pharma-quality capabilities,” said Pat McCutcheon, CEO, MediPharm Labs. “We have a very clear roadmap for international expansion and now the means to begin achieving our objectives in a risk-managed but assertive fashion.”
About VIVO Cannabis
VIVO Cannabis™ is recognized for trusted, premium cannabis products and services. It holds production and sales licences from Health Canada and operates world-class indoor and seasonal airhouse cultivation facilities with proprietary plant-growing technology in Hope, British Columbia and Napanee, Ontario. VIVO has a collection of premium brands, each targeting different customer segments, including Canna Farms™, Beacon Medical™, Fireside™, Lumina™ and Canadian Bud Collection™. VIVO Cannabis is expanding its production capabilities and distribution network. Harvest Medicine, VIVO’s patient-centric, scalable network of medical cannabis clinics, has serviced over 100,000 patient visits. VIVO is pursuing several partnership and product development opportunities and is focusing its international efforts on Germany and Australia.
About MediPharm Labs
Founded in 2015, MediPharm Labs specializes in the production of purified, pharmaceutical-quality cannabis oil and concentrates and advanced derivative products utilizing a Good Manufacturing Practices certified facility with ISO standard-built clean rooms. MediPharm Labs has invested in an expert, research driven team, state-of-the-art technology, downstream purification methodologies and purpose-built facilities with five primary extraction lines for delivery of pure, trusted and precision-dosed cannabis products for its customers. Through its wholesale and white label platforms, MediPharm Labs formulates, develops (including through sensory testing), processes, packages and distributes cannabis extracts and advanced cannabinoid-based products to domestic and international markets. As a global leader, MediPharm Labs has completed commercial exports to Australia and is nearing commercialization of its Australian extraction facility. MediPharm Labs Australia was established in 2017.
For further information, please contact:
Laura Lepore, VP, Investor Relations and Communications
Telephone: 416-913-7425 ext. 1525
Email: investors@medipharmlabs.com
Website: www.medipharmlabs.com
CAUTIONARY NOTE REGARDING FORWARD-LOOKING INFORMATION:
This news release contains “forward-looking information” and “forward-looking statements” (collectively, “forward-looking statements”) within the meaning of the applicable Canadian securities legislation. All statements, other than statements of historical fact, are forward-looking statements and are based on expectations, estimates and projections as at the date of this news release. Any statement that involves discussions with respect to predictions, expectations, beliefs, plans, projections, objectives, assumptions, future events or performance (often but not always using phrases such as “expects”, or “does not expect”, “is expected”, “anticipates” or “does not anticipate”, “plans”, “budget”, “scheduled”, “forecasts”, “estimates”, “believes” or “intends” or variations of such words and phrases or stating that certain actions, events or results “may” or “could”, “would”, “might” or “will” be taken to occur or be achieved) are not statements of historical fact and may be forward-looking statements. Forward-looking statements are necessarily based upon a number of estimates and assumptions that, while considered reasonable, are subject to known and unknown risks, uncertainties, and other factors which may cause the actual results and future events to differ materially from those expressed or implied by such forward-looking statements. Such factors include, but are not limited to: general business, economic, competitive, political and social uncertainties; the inability of MediPharm Labs to obtain adequate financing; the delay or failure to receive regulatory approvals; and other factors discussed in MediPharm Labs’ filings, available on the SEDAR website at www.sedar.com. There can be no assurance that such statements will prove to be accurate, as actual results and future events could differ materially from those anticipated in such statements. Accordingly, readers should not place undue reliance on the forward-looking statements and information contained in this news release. Except as required by law, MediPharm Labs assumes no obligation to update the forward-looking statements of beliefs, opinions, projections, or other factors, should they change.
1 “Access to medicinal cannabis products”; Australian Government, Department of Health, Therapeutic Goods Administration; Accessed online June 18, 2020: https://www.tga.gov.au/access-medicinal-cannabis-products-1.
2 “Australia’s legal cannabis market blooms to $1.5 billion by 2025”; April 12, 2020; Consultancy.com.au; Accessed online June 18, 2020: https://www.consultancy.com.au/news/1887/australias-legal-cannabis-market-blooms-to-15-billion-by-2025.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/0bb41b26-2167-49e2-8eef-4a9286de0afd