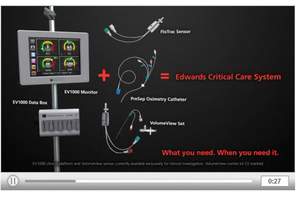
IRVINE, CA--(Marketwire - November 08, 2010) - Edwards Lifesciences Corporation (NYSE: EW), the global leader in the science of heart valves and hemodynamic monitoring, today announced a CE Mark for its new VolumeView sensor-catheter set that measures a patient's volumetric hemodynamic parameters and provides more clarity for clinicians treating critically ill patients. The company also announced a CE Mark for its new EV1000 clinical monitoring platform that integrates many of its sensors and catheters into one intuitive system. With a touch-screen monitor that displays a patient's physiologic status, as well as color-coded clinical targets and alerts, the EV1000 clinical platform is designed to simplify decision-making in the operating room (OR) and intensive care unit (ICU).
"The VolumeView set and EV1000 clinical platform present hemodynamic information in a new way that provides clarity and context to a patient's condition, and helps critical care clinicians with the many decisions they make for their patients, particularly those suffering from respiratory and circulatory failure," said Dr. Christoph Hofer, Triemli City Hospital Zurich, Switzerland. "It is a great advantage to be able to glean more patient insight as a result of a choice of parameters and multiple data viewing options when managing a variety of patient challenges."
"The innovative technology in our VolumeView set, combined with the intuitive EV1000 clinical platform, strengthens our product offering in the medical ICU," said Carlyn D. Solomon, corporate vice president, critical care of Edwards Lifesciences. "These innovations are representative of our commitment to meaningfully advance patient care and hemodynamic monitoring, a field we established with Drs. Swan and Ganz 40 years ago with the development of the Swan-Ganz catheter."
About the VolumeView Set
When used with the EV1000 clinical platform, the VolumeView set's sensor and catheter measure cardiac output and a range of volumetric parameters, including fluid volume in the lungs and global end-diastolic volume. These parameters are particularly valuable for clinicians in the medical ICU where more than two million patients globally experience respiratory and/or circulatory failure and could benefit from this technology. Additional information about the VolumeView set can be found at www.edwards.com/eu/VolumeView.
About the EV1000 Clinical Platform
Patient management using information provided by hemodynamic monitoring in the OR and ICU has been clinically proven to improve patient outcomes. It is estimated that more than four million patients globally could benefit from hemodynamic monitoring; however, this practice is very complex. To simplify the display and retrieval of hemodynamic information and expand its applicability, the EV1000 clinical platform allows patient data to be displayed via several different screen options. These screens utilize color-coding to reflect patient status. The company's FloTrac sensor, PediaSat and PreSep oximetry catheters, TruWave disposable pressure transducer, and VolumeView set are compatible with this platform. Additional information about the EV1000 clinical platform can be found at www.edwards.com/eu/EV1000.
Dr. Hofer is a principal investigator for a clinical evaluation of the VolumeView set and the EV1000 clinical platform, and provides paid consulting services to Edwards as an educator for critical care technologies.
About Edwards Lifesciences
Edwards Lifesciences is the global leader in the science of heart valves and hemodynamic monitoring. Driven by a passion to help patients, the company partners with clinicians to develop innovative technologies in the areas of structural heart disease and critical care monitoring that enable them to save and enhance lives. Additional company information can be found at www.edwards.com.
Edwards, EV1000 and VolumeView are trademarks of Edwards Lifesciences Corporation; Edwards Lifesciences, the stylized E logo, FloTrac, PediaSat, PreSep, TruWave and Swan-Ganz are trademarks of Edwards Lifesciences Corporation and are registered in the United States Patent and Trademark Office.
Media Contact:
Amanda C. Fowler
949-250-5070
Investor Contact:
David K. Erickson
949-250-6826