| | – PRGN-3005 was well-tolerated with no dose limiting toxicities, no CRS greater than Grade 2, and no neurotoxicity –
– PRGN-3005 cells demonstrated expansion and persistence when delivered via either intraperitoneal or intravenous infusion without lymphodepletion or via intravenous infusion after lymphodepletion demonstrating the effectiveness of mbIL15 –
– A single intravenous infusion following lymphodepletion decreased tumor burden in 67% of the heavily pretreated patients (median of 8 or more prior therapies) with 90% of individual target lesions showing stable disease or partial response –
– PRGN-3005 UltraCAR-T cells are being evaluated in the Phase 1b dose expansion study with intravenous infusion following lymphodepletion and incorporating repeat infusion –
– Best responder achieved stable disease for more than 18 months after failing 9 prior lines of treatment; results achieved with two doses of UltraCAR-T cells in the low millions – GERMANTOWN, Md., June 5, 2023 /PRNewswire/ -- Precigen, Inc. (Nasdaq: PGEN), a biopharmaceutical company specializing in the development of innovative gene and cell therapies to improve the lives of patients, today presented positive data at the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting from the Phase 1 portion of the Phase 1/1b clinical study evaluating safety and efficacy of PRGN-3005 UltraCAR-T® in advanced stage platinum resistant ovarian cancer patients (Abstract# 5590). The presentation was delivered by John B. Liao, MD, PhD, Cancer Vaccine Institute, University of Washington Medicine, and a lead investigator for the PRGN-3005 clinical trial. “We are pleased with the results of the Phase 1 study which demonstrate a favorable safety profile for PRGN-3005 UltraCAR-T. Our UltraCAR-T therapies continue to be well-tolerated with no dose limiting toxicities across our clinical stage UltraCAR-T portfolio,” said Helen Sabzevari, PhD, President and CEO of Precigen. “At this early stage, we are encouraged that patients in the IV plus lymphodepletion arm showed stable or partial response in 90% of the individual target lesions, and that the case study presented demonstrated that the repeat dosing further decreased tumor burden. UltraCAR-T is the only CAR-T platform that has demonstrated the ability to expand and persist and show a reduction in tumor burden and lesions with low doses of UltraCAR-T cells in this heavily pretreated ovarian cancer patient population. We are currently underway in the Phase 1b dose expansion study at Dose Level 3 via IV infusion with lymphodepletion and incorporating repeat dosing.” “Ovarian cancer symptoms are not always obvious and it is frequently diagnosed at an advanced stage where cancer has spread to distant parts of the body, which results in historically poor outcomes,” said Mary L. (Nora) Disis, MD, faculty member at the University of Washington and Fred Hutchinson Cancer Research Center and one of the lead investigators for the PRGN-3005 study. “The patients enrolled were heavily pretreated with a median of 8 or more prior lines of therapy and had significantly advanced stage disease with a high baseline tumor burden and distant metastases. PRGN-3005 resulted in a decrease in tumor burden in 67% of patients who received just one IV infusion following lymphodepletion. We are encouraged by the expansion, persistence and activity of the UltraCAR-T cells manufactured using the overnight process in this heavily pre-treated population of the patients, and in view of the favorable safety profile and preliminary activity, we are looking forward to utilizing PRGN-3005 cells in patients with fewer prior lines of therapy for their disease.” PRGN-3005 UltraCAR-T is an autologous chimeric antigen receptor T (CAR-T) cell therapy manufactured using non-viral gene delivery and is under investigation for the treatment of patients with advanced, recurrent platinum resistant ovarian, fallopian tube or primary peritoneal cancer. PRGN-3005 utilizes Precigen’s transformative UltraCAR-T therapeutic platform, which eliminates ex vivo expansion and reduces manufacturing time to allow for rapid next day administration of UltraCAR-T cells following non-viral gene transfer. PRGN-3005 UltraCAR-T is a multigenic CAR-T cell investigational therapy utilizing Precigen’s advanced non-viral gene delivery system to co-express a chimeric antigen receptor, membrane-bound interleukin‐15 (mbIL15), and a kill switch for better precision and control. Conducted in collaboration with the University of Washington and Fred Hutchinson Cancer Research Center, the Phase 1 study has completed dose escalation, which evaluated the safety and efficacy of PRGN-3005 administered either via intraperitoneal (IP) or intravenous (IV) infusion, in the absence of lymphodepletion (LD) or IV following lymphodepletion with cyclophosphamide only (clinical trial identifier: NCT03907527). Patient Demographics
The study population includes patients with advanced stage (III/IV) recurrent ovarian, fallopian tube, and primary peritoneal cancer who are platinum resistant and have progressed after receiving standard-of-care therapies or are not eligible to receive available therapies with known clinical benefit (Table 1). The mean age of patients in the study was 57.4 in the IP arm without lymphodepletion, 67.3 in the IV arm without lymphodepletion and 62.7 in the IV arm with lymphodepletion. Patients were heavily pretreated with a median of greater than or equal to 8 prior lines of therapy across all arms. Patients had significantly advanced stage disease with a high baseline tumor burden with most patients having distant metastases, including liver, spleen, bladder and lung. Table 1: Phase 1 Dose Escalation Patient Demographics | | IP (N=12) | IV (N=6) | IV with LD (N=9) | | Age (years) | | | | | | Mean, Median | 57.4, 59.5 | 67.3, 69.5 | 62.7, 64 | | | Range | 38-70 | 54-76 | 47-77 | | ECOG Status | | | | | | 0 | 4 (33.3 %) | 3 (50 %) | 5 (55.6 %) | | | 1 | 8 (66.7 %) | 3 (50 %) | 4 (44.4 %) | | Baseline Tumor Burden (mm) | | | | | | Mean, Median | 71.2, 54.3 | 61.6, 46 | 97.1, 109.5 | | | Range | 15.2- 142.8 | 33.7 -145.7 | 44.5 - 149.8 | | Baseline CA 125 | | | | | | Mean, Median | 1845.2, 1208.5 | 789.2, 199.5 | 539.3, 486 | | | Range | 82-8148 | 42-3035 | 60-1301 | | Prior lines of therapy | | | | | Mean, median (range) | 8.2, 8.5 (5-11) | 7.7, 8 (4-10) | 7.7, 8 (5-11) | | | 4-5 | 2 (16.7 %) | 1 (16.7 %) | 2 (22.2 %) | | | 6-11 | 10 (83.3 %) | 5 (83.3 %) | 7 (77.8 %) | Safety Data
PRGN-3005 was well-tolerated with low incidence of treatment related adverse events (TRAEs), no dose limiting toxicities (DLTs), and no neurotoxicity (Table 2). The most common side effects for the IV and IP arms without lymphodepletion were abdominal pain, fever and decreased absolute lymphocyte count (ALC). Serious Adverse Events included five incidences of Cytokine Release Syndrome (CRS), with no incidence of CRS greater than Grade 2. One patient with CRS required specific intervention which resolved following standard CRS management after 24 hours. There was no use of tocilizumab or dexamethasone or kill switch. Table 2: PRGN-3005 Treatment Related Adverse Events | | IP (N=12) | IV (N=6) | IV with LD (N=9) | | | # of
Events | Patients (n, %) | # of
Events | Patients (n, %) | # of
Event | Patients (n, %) | | Abdominal Pain | 7 | 1 (8 %) | 0 | 0 | 0 | 0 | | Cytokine Release Syndrome | 0 | 0 | 1 | 1 (17 %) | 5 | 5 (56 %) | | Grade 1 CRS | 0 | 0 | 1 | 1 / 1 | 4 | 4 / 5 | | Grade 2 CRS | 0 | 0 | 0 | 0 / 1 | 1 | 1 / 5 | | Fever | 1 | 1 (8 %) | 1 | 1 (17 %) | 2 | 2 (22 %) | | Hypotension | 0 | 0 | 0 | 0 | 1 | 1 (11 %) | | ALC, Decreased | 6 | 2 (17 %) | 12 | 2 (33 %) | 0 | 0 | | Nausea | 0 | 0 | 0 | 0 | 1 | 1 (11 %) | | Small Intestinal Obstruction | 1 | 1 (8 %) | 0 | 0 | 0 | 0 | | White Blood Cell Decreased | 0 | 0 | 1 | 1 (17 %) | 1 | 1 (11 %) | | Summary of Events of Interest | | DLTs | 0 | 0 | 0 | 0 | 0 | 0 | | Serious Adverse Events | 6 | 5 (41 %) | 2 | 1 (17 %) | 4 | 4 (44 %) | | Neurotoxicity | 0 | 0 | 0 | 0 | 0 | 0 | | | | | | | | | | Clinical Activity Expansion Kinetics
PRGN-3005 administered either intraperitoneally or intravenously at doses as low as 2 million CAR-T cells resulted in a dose-dependent expansion and encouraging persistence in peripheral blood. Tumor Responses
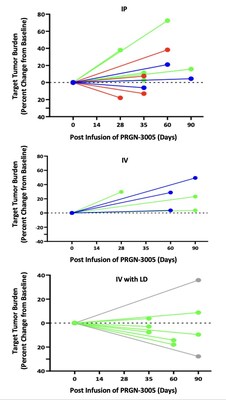
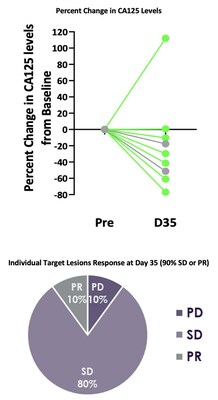
Best responses in patients treated without lymphodepletion were stable disease with complete responses in individual target lesions. Incorporating lymphodepletion prior to IV infusion led to an encouraging efficacy signal as demonstrated by a decrease in tumor burden in 67% (6/9) of patients (Figure 1), with concurrent decreases in CA125 at Day 35 in 89% (8/9) of patients and stable or partial response in 90% of the individual target lesions (Figure 2). Figure 1: Target Tumor Burden Figure 2: CA125 Levels and Target Lesion Response in the IV Administration with Lymphodepletion Arm Case Study
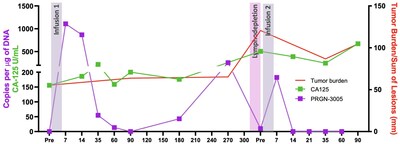
The best response was observed in a 73 year old female patient who was heavily pretreated with more than 9 prior lines of therapy. The patient received two infusions of PRGN-3005, one year apart. The first infusion of 2 million UltraCAR-T cells was administered via IV without lymphodepletion, after which the patient had durable stable disease for about 1 year. Subsequent to the increase in tumor burden, UltraCAR-T cells in the blood also expanded. The patient also received a second infusion of cells this time following lymphodepletion and demonstrated a 28% decrease in tumor burden. After the second infusion there was a significant increase in MUC16-specific T-cell activity and ongoing survival for more than 18 months (Figures 3, 4). Figure 3: Repeat Dosing and Tumor Response for Best Responder Figure 4: Expansion and Tumor Burden/Sum of Lesions for Best Responder PRGN-3005 UltraCAR-T cells are currently being evaluated in the Phase 1b dose expansion study at Dose Level 3 via IV infusion with lymphodepletion and incorporating repeat dosing. About Ovarian Cancer
Worldwide, nearly 300,000 women are diagnosed with ovarian cancer every year1 with approximately 22,000 of them in the US2. Since early ovarian cancer is often without obvious symptoms, the disease is frequently diagnosed at an advanced stage where cancer has spread to distant parts of the body, such as the liver or lungs2,3. Five-year survival rates depend on stage and type of ovarian cancer with rates decreasing for advanced stage cancers that have spread to distant parts of the body3. Precigen: Advancing Medicine with Precision™
Precigen (Nasdaq: PGEN) is a dedicated discovery and clinical stage biopharmaceutical company advancing the next generation of gene and cell therapies using precision technology to target the most urgent and intractable diseases in our core therapeutic areas of immuno-oncology, autoimmune disorders, and infectious diseases. Our technologies enable us to find innovative solutions for affordable biotherapeutics in a controlled manner. Precigen operates as an innovation engine progressing a preclinical and clinical pipeline of well-differentiated therapies toward clinical proof-of-concept and commercialization. For more information about Precigen, visit www.precigen.com or follow us on Twitter @Precigen, LinkedIn or YouTube. UltraCAR-T®
UltraCAR-T is a multigenic autologous CAR-T platform that utilizes Precigen’s advanced non-viral Sleeping Beauty system to simultaneously express an antigen-specific CAR to specifically target tumor cells, mbIL15 for enhanced in vivo expansion and persistence, and a kill switch to conditionally eliminate CAR-T cells for a potentially improved safety profile. Precigen has advanced the UltraCAR-T platform to address the inhibitory tumor microenvironment by incorporating a novel mechanism for intrinsic checkpoint blockade without the need for complex and expensive gene editing techniques. UltraCAR-T investigational therapies are manufactured via Precigen’s overnight manufacturing process using the proprietary UltraPorator® electroporation system at the medical center and administered to patients only one day following gene transfer. The overnight UltraCAR-T manufacturing process does not use viral vectors and does not require ex vivo activation and expansion of T cells, potentially addressing major limitations of current T cell therapies. UltraCAR-T® Clinical Program
The UltraCAR-T platform has shifted the autologous CAR-T manufacturing paradigm using an advanced non-viral multigene delivery system and an overnight, decentralized manufacturing process for administration of autologous CAR-T cells one day after gene transfer to reduce vein-to-vein time. Precigen’s UltraCAR-T platform is currently under clinical investigation for hematological and solid tumors, including a Phase 1/1b study of PRGN-3005 UltraCAR-T in patients with advanced, recurrent platinum resistant ovarian, fallopian tube or primary peritoneal cancer (NCT03907527), a Phase 1/1b study of PRGN-3006 UltraCAR-T in patients with relapsed or refractory acute myeloid leukemia (AML) or higher risk myelodysplastic syndrome (MDS) (NCT03927261) and a Phase 1/1b study of PRGN-3007 UltraCAR-T incorporating PD-1 checkpoint inhibition in patients with ROR1-positive (ROR1+) hematologic chronic lymphocytic leukemia (CLL), mantle cell lymphoma (MCL), acute lymphoblastic leukemia (ALL), diffuse large B-cell lymphoma (DLBCL) and solid tumor triple negative breast cancer (TNBC) malignancies (NCT05694364). PRGN-3006 UltraCAR-T has been granted Orphan Drug Designation and Fast Track Designation in patients with AML by the US Food and Drug Administration (FDA). UltraCAR-T® Library Approach
Precigen’s UltraCAR-T library approach is designed to transform the personalized cell therapy landscape for cancer patients. Precigen’s goal is to develop and validate a library of non-viral plasmids to target tumor-associated antigens. Enabled by design and manufacturing advantages of UltraCAR-T, coupled with the capabilities of the UltraPorator® system, Precigen is working to empower cancer centers to deliver personalized, autologous UltraCAR-T treatment with overnight manufacturing to any cancer patient. Based on the patient’s cancer indication and biomarker profile, one or more non-viral plasmids would be selected from the library to build a personalized UltraCAR-T treatment. After initial treatment, this approach has the potential to allow for redosing of UltraCAR-T targeting the same or new tumor-associated antigen(s) based on the treatment response and the changes in antigen expression of the patient’s tumor. Precigen believes that the combination of the advanced UltraVector® DNA construction platform and the ease of overnight manufacturing gives this library approach a proprietary advantage over traditional T-cell therapies. UltraPorator®
The UltraPorator system is an exclusive device and proprietary software solution for the scale-up of rapid and cost-effective manufacturing of UltraCAR-T therapies and potentially represents a major advancement over current electroporation devices by significantly reducing the processing time and contamination risk. The UltraPorator device is a high-throughput, semi-closed electroporation system for modifying T cells using Precigen’s proprietary non-viral gene transfer technology. UltraPorator is being utilized for clinical manufacturing of Precigen’s investigational UltraCAR-T therapies in compliance with current good manufacturing practices. Trademarks
Precigen, UltraCAR-T, UltraVector, UltraPorator, and Advancing Medicine with Precision are trademarks of Precigen and/or its affiliates. Other names may be trademarks of their respective owners. Cautionary Statement Regarding Forward-Looking Statements
Some of the statements made in this press release are forward-looking statements. These forward-looking statements are based upon the Company’s current expectations and projections about future events and generally relate to plans, objectives, and expectations for the development of the Company’s business, including the timing and progress of preclinical studies, clinical trials, discovery programs and related milestones, the promise of the Company’s portfolio of therapies, and in particular its CAR-T and AdenoVerse therapies. Although management believes that the plans and objectives reflected in or suggested by these forward-looking statements are reasonable, all forward-looking statements involve risks and uncertainties, including the possibility that the timeline for the Company’s clinical trials might be impacted by the COVID-19 pandemic, and actual future results may be materially different from the plans, objectives and expectations expressed in this press release. The Company has no obligation to provide any updates to these forward-looking statements even if its expectations change. All forward-looking statements are expressly qualified in their entirety by this cautionary statement. For further information on potential risks and uncertainties, and other important factors, any of which could cause the Company’s actual results to differ from those contained in the forward-looking statements, see the section entitled “Risk Factors” in the Company’s most recent Annual Report on Form 10-K and subsequent reports filed with the Securities and Exchange Commission. References
1World Health Organization, International Agency for Research on Cancer, Global Cancer Observatory. Cancer Today, Estimated number of new cases in 2018. Accessed via WHO IARC GCO website.
2American Cancer Society Ovarian Cancer Special Section. Accessed via ACS website.
3American Cancer Society. Survival Rates for Ovarian Cancer. Accessed via ACS website. Investor Contact:
Steven M. Harasym
Vice President, Investor Relations
Tel: +1 (301) 556-9850
investors@precigen.com Media Contacts:
Donelle M. Gregory
press@precigen.com Glenn Silver
Lazar-FINN Partners
glenn.silver@finnpartners.com  View original content to download multimedia:https://www.prnewswire.com/news-releases/precigen-announces-positive-phase-1-data-for-prgn-3005-autologous-ultracar-t-cells-manufactured-overnight-for-infusion-next-day-to-advanced-stage-platinum-resistant-ovarian-cancer-patients-301842691.html View original content to download multimedia:https://www.prnewswire.com/news-releases/precigen-announces-positive-phase-1-data-for-prgn-3005-autologous-ultracar-t-cells-manufactured-overnight-for-infusion-next-day-to-advanced-stage-platinum-resistant-ovarian-cancer-patients-301842691.html
SOURCE Precigen, Inc. | |