Study to enroll 144 patients with COVID-19 who have progressed to Acute Respiratory Distress and require intensive care with mechanical ventilation
- Study to enroll 144 patients with COVID-19 who have progressed to Acute Respiratory Distress and require intensive care with mechanical ventilation
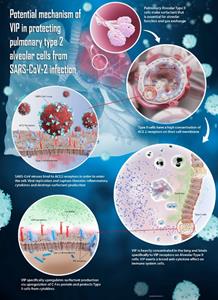
- RLF-100 is a patented formulation of Aviptadil (synthetic human Vasoactive Intestinal Polypeptide), which binds to alveolar type 2 cells in the lungs. Type 2 cells are essential to oxygen exchange and are selectively targeted by the SARS-CoV-2 virus
GENEVA and RADNOR, Pa., May 14, 2020 (GLOBE NEWSWIRE) -- RELIEF THERAPEUTICS Holding AG (SIX:RLF) (Relief) and its U.S. partner, NeuroRx, Inc. announced today that NeuroRx has completed final manufacturing of investigational drug RLF-100 for Phase 2b/3 clinical trial to assess intravenous RLF-100 as a treatment for Acute Respiratory Distress Syndrome (ARDS) in COVID-19 patients on mechanical ventilation.
Bachem Americas (Torrance, Calif.) and MedisourceRx (Encinatas, Calif.) respectively, manufactured RLF-100 drug substance and drug product under the supervision of NeuroRx scientists Richard Siegel, Ph.D., and Robert Besthof MIM, who previously held leadership positions at Johnson & Johnson and Pfizer prior to joining NeuroRx. Manufacture and release of investigational product was completed within 60 days of FDA’s approval to proceed with the clinical trial.
Researchers at the University of Miami have begun enrollment of patients with COVID-19 and enrollment at Thomas Jefferson University, and University of California – Irvine is expected to commence shortly. Additional sites at NYU Langone and six other locations across the U.S. will begin enrolling patients by June 2020. RLF-100 is a patented formulation of Aviptadil, a synthetic human vasoactive intestinal polypeptide (VIP), that targets alveolar type 2 cells in the lungs attacked by SARS-CoV-2 virus. VIP is known from numerous animal models of lung injury and lung disease to inhibit inflammatory cytokines and to protect pulmonary epithelial cells that line the air sacs (alveolae) of the lungs.
“Tragically, survival of patients with COVID-19 who progress to Acute Respiratory Distress is dismal. There is an urgent need for a treatment approach that goes right into the heart of the matter – the alveolar type 2 cells which are vulnerable entry points and hosts for the SARS-CoV-2 virus,” said Jonathan Javitt, M.D., CEO of NeuroRx. “In previous human studies, RLF-100 was seen to decrease inflammatory cytokines. In laboratory studies, it is known to increase the production of surfactants by type 2 cells needed to maintain oxygen exchange in the lungs and could potentially protect type 2 cells from cell death in COVID-19.”
RLF-100 is a synthetic form of VIP, which is the body’s defense against many lung injuries ranging from infectious disease to smoke inhalation. VIP is known to preferentially bind to the precious type 2 cells in the lungs that are directly attacked by the coronavirus. While there are many drugs being tested in the clinic today, we are not aware of another drug candidate that specifically targets the cells that are lost in COVID-19 lung injury.
Relief Therapeutics and NeuroRx are engaging clinical trials authorities in the European Union, the United Kingdom, Russia, and Australia in order to broaden the clinical study and increase access to RLF-100.
About VIP in Lung Injury
Vasoactive Intestinal Polypeptide (VIP) was first characterized by the late Dr. Sami Said in the 1970s. Although first identified in the intestinal tract, VIP is now known to be produced throughout the body and to be heavily concentrated in the lungs. VIP has been shown in more than 100 peer-reviewed studies to have potent anti-inflammatory/anti-cytokine activity in animal models of respiratory distress, acute lung injury, and inflammation. VIP has a 20-year history of safe use in humans in multiple human trials for sarcoidosis, pulmonary fibrosis, asthma/allergy, and pulmonary hypertension.
COVID-19-related death is primarily caused by Acute Respiratory Distress Syndrome (ARDS). The trigger for ARDS is widely attributed to a cytokine storm in the lungs, in which the virus causes release of inflammatory molecules called cytokines. As a result, the air sacs (alveolae) of the lungs fill with water and become impermeable to oxygen, even in the setting of mechanical ventilation. Before this acute phase, however, there is evidence of early viral infection of the alveolar type 2 cells.1 These cells are known to have angiotensin converting enzyme 2 (ACE2) receptors at high levels, which serve as the route of entry for the SARS-CoV-2 into the cells. Although not yet shown for COVID-19, the coronavirus that causes SARS (SARS-CoV) is shown to replicate in alveolar type 2 cells, but not in the more numerous type 1 cells. 2 These same type 2 alveolar cells have high concentrations of VIP receptors on their cell surfaces giving rise to the hypothesis that VIP could specifically protect these cells from injury.
Injury to the type 2 alveolar cells is an increasingly plausible mechanism of COVID-19 disease progression. These specialized cells replenish the more common type 1 cells that line the lungs. More importantly, type 2 cells manufacture surfactant that coats the lung and are essential for oxygen exchange. Patients with early COVID-19 lung injury commonly describe “crackling sounds” in their lungs, combined with extreme shortness of breath. No currently proposed treatments for COVID-19 specifically target these vulnerable type 2 cells.
About RELIEF THERAPEUTICS Holding AG
The Relief group of companies focus primarily on clinical-stage projects based on molecules of natural origin (peptides and proteins) with a history of clinical testing and use in human patients or a strong scientific rational. Currently, Relief is concentrating its efforts on developing new treatments for respiratory disease indications.
About NeuroRx, Inc.
NeuroRx draws upon more than 100 years of collective drug development experience and is led by former senior executives of Johnson & Johnson, BMS, Eli Lilly, Pfizer, and Sunovion. In addition to its work on RLF-100, NeuroRx has been awarded Breakthrough Therapy Designation and a Special Protocol Agreement to develop NRX-101 for the treatment of suicidal bipolar depression and is currently in Phase 3 trials. Its Board of Directors and Advisors includes Hon. Sherry Glied, former Assistant Secretary, U.S. Dept. of Health and Human Services; Mr. Chaim Hurvitz, former President of the Teva International Group, Lt. Gen. HR McMaster, the 23rd National Security Advisor, Wayne Pines, former Associate Commissioner of the U.S. Food and Drug Administration, Judge Abraham Sofaer, and Daniel Troy, former Chief Counsel, U.S. Food and Drug Administration.
About RLF-100
RLF-100 (Aviptadil) is a patented formulation of Vasoactive Intestinal Polypeptide (VIP) that was originally developed and is currently marketed in Europe for the treatment of erectile dysfunction. VIP is known to be highly concentrated in the lungs and to inhibit a variety of inflammatory cytokines. Relief’s predecessor company, Mondo Biotech, was awarded Orphan Drug Designation in 2001 by the U.S. FDA for Aviptadil in the treatment of Acute Respiratory Distress Syndrome and in 2005 for treatment of Pulmonary Arterial Hypertension. Mondo was awarded Orphan Drug Designation by the European Medicines Agency in 2006 for the treatment of acute lung injury and in 2007 for the treatment of sarcoidosis. Both the U.S. FDA and the EMEA have granted Investigational New Drug licenses for human trials of Aviptadil.
RELIEF THERAPEUTICS Holding AG is listed on the SIX Swiss Exchange under the symbol RLF.
Disclaimer: This communication expressly or implicitly contains certain forward-looking statements concerning RELIEF THERAPEUTICS Holding AG, NeuroRx, Inc. and their businesses. Such statements involve certain known and unknown risks, uncertainties and other factors, which could cause the actual results, financial condition, performance or achievements of RELIEF THERAPEUTICS Holding AG and/or NeuroRx, Inc. to be materially different from any future results, performance or achievements expressed or implied by such forward-looking statements. RELIEF THERAPEUTICS Holding AG is providing this communication as of this date and does not undertake to update any forward-looking statements contained herein as a result of new information, future events or otherwise.
CORPORATE CONTACTS
Jonathan C. Javitt, M.D., MPH
CEO
NeuroRx, Inc.
ceo@neurorxpharma.com
Yves Sagot, Ph.D.
Chief Scientist
Relief Therapeutics Holding, SA
yves.sagot@relieftherapeutics.com
MEDIA CONTACT
Gloria Gasaatura
LifeSci Communications
ggasaatura@lifescicomms.com
646-970-4688
1 Mason R. Pathogenesis of COVID-19 from a cell biologic perspective. Eur Respir J. April 9 Epub ahead of print. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7144260/
2 Moseel EC, Wang J, Jeffers S, et. al. SARS-CoV replicates in primary human alveolar type II cell cultures but not in type 1-like cells. Virology 2008;372(1):127-135 https://pubmed.ncbi.nlm.nih.gov/18022664/
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/82ac9acb-fa51-48d7-8c0d-9883aaf43578