Organon & Co. & Samsung Bioepis Co., Ltd. announced that HADLIMA™, a biosimilar referencing Humira, is now available to patients in the United States.
- Autoinjector recognized by the Arthritis Foundation with Ease of Use Certification
- Individualized patient support program “HADLIMA For You” available including co-pay support
- Designed to improve access and affordability for patients and the US health care system
JERSEY CITY, N.J. & INCHEON, Korea--(BUSINESS WIRE)-- Organon & Co. (NYSE: OGN) & Samsung Bioepis Co., Ltd. announced that HADLIMA™ (adalimumab-bwwd), a biosimilar referencing Humira (adalimumab), is now available to patients in the United States. Consistent with Humira, HADLIMA is available in both citrate-free high concentration (100 mg/mL) and citrate-containing low concentration (50 mg/mL) to provide patients with seamless continuity of care.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20230701798272/en/
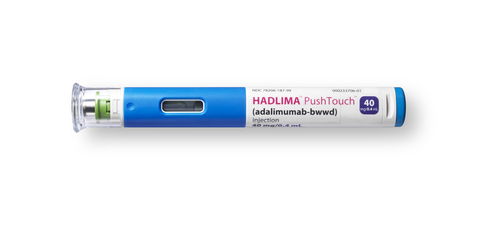
HADLIMA (adalimumab-bwwd) PushTouch Autoinjector Close-up (Photo: Business Wire)
“As the largest loss-of-exclusivity event in pharmaceutical history, this is a singular moment for the US health care system to embrace biosimilars. Every stakeholder should be invested in the success of this market to realize the value biosimilars can create for patients, providers, and the US health care economy,” said Kevin Ali, Chief Executive Officer of Organon. “We are thrilled to now provide HADLIMA in the US at a more affordable cost and expand much-needed access to adalimumab. With our deep biosimilar commercial experience, a new comprehensive patient support program, and our dedication to providing exceptional HCP support, we are immediately well-positioned to make a positive impact.”
“The availability of HADLIMA, both high and low concentration, marks an important milestone towards expanding treatment options for millions of patients suffering from chronic autoimmune diseases in the United States. Based on our robust track record over the past four years with approximately 6.8 million units of our adalimumab biosimilar supplied in ex-US markets, we are well-positioned to deliver this life-changing medicine to patients through stringent quality control, rigorous manufacturing, and supply resilience,” said Christopher Hansung Ko, President and Chief Executive Officer of Samsung Bioepis. “Our mission is to positively impact and ensure the sustainability of health care systems by offering affordable, clinically proven biologic medicines. We will continue to work with our partners and other stakeholders to ensure wider availability of this medicine in the US,” he added.
HADLIMA is a tumor necrosis factor (TNF) blocker indicated for appropriate patients with rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, and plaque psoriasis. Patients treated with adalimumab products, including HADLIMA, are at increased risk for developing serious infections that may lead to hospitalization or death. Discontinue HADLIMA if a patient develops a serious infection or sepsis. Monitor patients closely for the development of signs and symptoms of infection during and after treatment with HADLIMA, including the possible development of tuberculosis (TB) in patients who tested negative for latent TB infection prior to initiating therapy. Lymphoma and other malignancies, some fatal, have been reported in children and adolescent patients treated with TNF blockers including adalimumab products. See additional safety information below.
HADLIMA (a carton including two pre-filled pens or two pre-filled syringes) is available at a list price (wholesale acquisition cost) of $1,038, which represents an 85% discount in comparison to the list price of Humira, in order to enable expanded access to patients.
Samsung Bioepis’s adalimumab biosimilar (also known as SB5, marketed under different brand names ex-US) has been available in 24 markets globally since 2018 and is supported by extensive clinical experience across rheumatologic, dermatologic and gastroenterological conditions. SB5 has also been evaluated in more than 20 real-world studies including data from over 5,100 patients.
Autoinjector Option Recognized by the Arthritis Foundation
HADLIMA is available in both a pre-filled syringe and PushTouch autoinjector option. The autoinjector was specifically designed with the patient in mind, with a thin 29G needle, a latex-free needle cover, and a buttonless device with a sure-grip shape and a non-slip surface that is intended to fit into a patient’s lifestyle. The autoinjector has been awarded the Arthritis Foundation’s Ease of Use Certification, which recognizes products that make life easier for those living with arthritis and other functional limitations via lab and patient testing by the Intuitive Design Applied Research Institute (IDARI).1
Individualized Patient Support Program
The “HADLIMA For You” patient support program features comprehensive educational resources including a co-pay program and dedicated nurse coaches who will be available to engage with patients throughout their treatment journey. Each registered nurse will also be certified as a health coach to provide an experience tailored to fit the unique needs of each patient. More information can be found at www.HADLIMA.com.
About HADLIMA™ (adalimumab-bwwd) Injection 40 mg/0.4 mL and 40 mg/0.8mL
HADLIMA is a tumor necrosis factor (TNF) blocker indicated for:
- Rheumatoid Arthritis: HADLIMA is indicated, alone or in combination with methotrexate or other non-biologic disease-modifying antirheumatic drugs (DMARDs), for reducing signs and symptoms, inducing major clinical response, inhibiting the progression of structural damage, and improving physical function in adult patients with moderately to severely active rheumatoid arthritis.
- Juvenile Idiopathic Arthritis: HADLIMA is indicated, alone or in combination with methotrexate, for reducing signs and symptoms of moderately to severely active polyarticular juvenile idiopathic arthritis in patients 2 years of age and older.
- Psoriatic Arthritis: HADLIMA is indicated, alone or in combination with non-biologic DMARDs, for reducing signs and symptoms, inhibiting the progression of structural damage, and improving physical function in adult patients with active psoriatic arthritis.
- Ankylosing Spondylitis: HADLIMA is indicated for reducing signs and symptoms in adult patients with active ankylosing spondylitis.
- Crohn’s Disease: HADLIMA is indicated for the treatment of moderately to severely active Crohn’s disease in adults and pediatric patients 6 years of age and older.
- Ulcerative Colitis: HADLIMA is indicated for the treatment of moderately to severely active ulcerative colitis in adult patients.
Limitations of Use:
The effectiveness of HADLIMA has not been established in patients who have lost response to or were intolerant to tumor necrosis factor (TNF) blockers.
- Plaque Psoriasis: HADLIMA is indicated for the treatment of adult patients with moderate to severe chronic plaque psoriasis who are candidates for systemic therapy or phototherapy, and when other systemic therapies are medically less appropriate. HADLIMA should only be administered to patients who will be closely monitored and have regular follow-up visits with a physician.
SELECTED SAFETY INFORMATION
SERIOUS INFECTIONS
Patients treated with adalimumab products, including HADLIMA, are at increased risk for developing serious infections that may lead to hospitalization or death. Most patients who developed these infections were taking concomitant immunosuppressants such as methotrexate or corticosteroids.
Discontinue HADLIMA if a patient develops a serious infection or sepsis.
Reported infections include:
- Active tuberculosis (TB), including reactivation of latent TB. Patients with TB have frequently presented with disseminated or extrapulmonary disease. Test patients for latent TB before HADLIMA use and during therapy. Initiate treatment for latent TB prior to HADLIMA use.
- Invasive fungal infections, including histoplasmosis, coccidioidomycosis, candidiasis, aspergillosis, blastomycosis, and pneumocystosis. Patients with histoplasmosis or other invasive fungal infections may present with disseminated, rather than localized, disease. Antigen and antibody testing for histoplasmosis may be negative in some patients with active infection. Consider empiric anti-fungal therapy in patients at risk for invasive fungal infections who develop severe systemic illness.
- Bacterial, viral, and other infections due to opportunistic pathogens, including Legionella and Listeria.
Carefully consider the risks and benefits of treatment with HADLIMA prior to initiating therapy in patients:
- with chronic or recurrent infection
- who have been exposed to TB
- with a history of opportunistic infection
- who resided in or traveled in regions where mycoses are endemic
- with underlying conditions that may predispose them to infection
Monitor patients closely for the development of signs and symptoms of infection during and after treatment with HADLIMA, including the possible development of TB in patients who tested negative for latent TB infection prior to initiating therapy.
- Do not start HADLIMA during an active infection, including localized infections.
- Patients older than 65 years, patients with co-morbid conditions, and/or patients taking concomitant immunosuppressants may be at greater risk of infection.
- If an infection develops, monitor carefully and initiate appropriate therapy.
- Drug interactions with biologic products: A higher rate of serious infections has been observed in rheumatoid arthritis (RA) patients treated with rituximab who received subsequent treatment with a TNF blocker. An increased risk of serious infections has been seen with the combination of TNF blockers with anakinra or abatacept, with no demonstrated added benefit in patients with RA. Concomitant administration of HADLIMA with other biologic DMARDs (e.g., anakinra or abatacept) or other TNF blockers is not recommended based on the possible increased risk for infections and other potential pharmacological interactions.
MALIGNANCY
Lymphoma and other malignancies, some fatal, have been reported in children and adolescent patients treated with TNF blockers, including adalimumab products. Postmarketing cases of hepatosplenic T-cell lymphoma (HSTCL), a rare type of T-cell lymphoma, have been reported in patients treated with TNF blockers, including adalimumab products. These cases have had a very aggressive disease course and have been fatal. The majority of reported TNF blocker cases have occurred in patients with Crohn’s disease or ulcerative colitis and the majority were in adolescent and young adult males. Almost all of these patients had received treatment with azathioprine or 6-mercaptopurine concomitantly with a TNF blocker at or prior to diagnosis. It is uncertain whether the occurrence of HSTCL is related to use of a TNF blocker or a TNF blocker in combination with these other immunosuppressants.
- Consider the risks and benefits of HADLIMA treatment prior to initiating or continuing therapy in a patient with known malignancy.
- In clinical trials, more cases of malignancies were observed among adalimumab-treated patients compared to control patients.
- Non-melanoma skin cancer (NMSC) was reported during clinical trials for adalimumab-treated patients. Examine all patients, particularly those with a history of prolonged immunosuppressant or psoralen and ultraviolet A (PUVA) therapy, for the presence of NMSC prior to and during treatment with HADLIMA.
- In adalimumab clinical trials, there was an approximate 3-fold higher rate of lymphoma than expected in the general U.S. population. Patients with chronic inflammatory diseases, particularly those with highly active disease and/or chronic exposure to immunosuppressant therapies, may be at higher risk of lymphoma than the general population, even in the absence of TNF blockers.
- Postmarketing cases of acute and chronic leukemia were reported with TNF blocker use. Approximately half of the postmarketing cases of malignancies in children, adolescents, and young adults receiving TNF blockers were lymphomas; other cases included rare malignancies associated with immunosuppression and malignancies not usually observed in children and adolescents.
HYPERSENSITIVITY
Anaphylaxis and angioneurotic edema have been reported following adalimumab administration. If a serious allergic reaction occurs, stop HADLIMA and institute appropriate therapy.
HEPATITIS B VIRUS REACTIVATION
Use of TNF blockers, including HADLIMA, may increase the risk of reactivation of hepatitis B virus (HBV) in patients who are chronic carriers. Some cases have been fatal.
Evaluate patients at risk for HBV infection for prior evidence of HBV infection before initiating TNF blocker therapy.
Exercise caution in patients who are carriers of HBV and monitor them during and after HADLIMA treatment.
Discontinue HADLIMA and begin antiviral therapy in patients who develop HBV reactivation. Exercise caution when resuming HADLIMA after HBV treatment.
NEUROLOGIC REACTIONS
TNF blockers, including adalimumab products, have been associated with rare cases of new onset or exacerbation of central nervous system and peripheral demyelinating diseases, including multiple sclerosis, optic neuritis, and Guillain-Barré syndrome.
Exercise caution when considering HADLIMA for patients with these disorders; discontinuation of HADLIMA should be considered if any of these disorders develop.
HEMATOLOGIC REACTIONS
Rare reports of pancytopenia, including aplastic anemia, have been reported with TNF blockers. Medically significant cytopenia has been infrequently reported with adalimumab products.
Consider stopping HADLIMA if significant hematologic abnormalities occur.
CONGESTIVE HEART FAILURE
Worsening and new onset congestive heart failure (CHF) has been reported with TNF blockers. Cases of worsening CHF have been observed with adalimumab products; exercise caution and monitor carefully.
AUTOIMMUNITY
Treatment with adalimumab products may result in the formation of autoantibodies and, rarely, in development of a lupus-like syndrome. Discontinue treatment if symptoms of a lupus-like syndrome develop.
IMMUNIZATIONS
Patients on HADLIMA should not receive live vaccines.
Pediatric patients, if possible, should be brought up to date with all immunizations before initiating HADLIMA therapy.
Adalimumab is actively transferred across the placenta during the third trimester of pregnancy and may affect immune response in the in utero-exposed infant. The safety of administering live or live-attenuated vaccines in infants exposed to adalimumab products in utero is unknown. Risks and benefits should be considered prior to vaccinating (live or live-attenuated) exposed infants.
ADVERSE REACTIONS
The most common adverse reactions in adalimumab clinical trials (>10%) were: infections (eg, upper respiratory, sinusitis), injection site reactions, headache, and rash.
Before prescribing HADLIMA, please read the accompanying Prescribing Information, including the Boxed Warning about serious infections and malignancies. The Medication Guide and Instructions for Use also are available.
About Organon
Organon is a global health care company formed to focus on improving the health of women throughout their lives. Organon offers more than 60 medicines and products in women’s health in addition to a growing biosimilars business and a large franchise of established medicines across a range of therapeutic areas. Organon’s existing products produce strong cash flows that support investments in innovation and future growth opportunities in women’s health and biosimilars. In addition, Organon is pursuing opportunities to collaborate with biopharmaceutical innovators looking to commercialize their products by leveraging its scale and presence in fast growing international markets. Organon has a global footprint with significant scale and geographic reach, world-class commercial capabilities, and approximately 10,000 employees with headquarters located in Jersey City, New Jersey.
For more information, visit http://www.organon.com and connect with us on LinkedIn and Instagram.
About Samsung Bioepis Co., Ltd.
Established in 2012, Samsung Bioepis is a biopharmaceutical company committed to realizing health care that is accessible to everyone. Through innovations in product development and a firm commitment to quality, Samsung Bioepis aims to become the world’s leading biopharmaceutical company. Samsung Bioepis continues to advance a broad pipeline of biosimilar candidates that cover a spectrum of therapeutic areas, including immunology, oncology, ophthalmology, hematology, and endocrinology. For more information, please visit: www.samsungbioepis.com and follow us on social media – Twitter, LinkedIn.
About the Organon-Samsung Bioepis Collaboration
HADLIMA is developed, manufactured and supplied by Samsung Bioepis, and commercialized by Organon. Samsung Bioepis and Organon have development and commercialization collaborations for two immunology products and one oncology product in the United States.
ORGANON, the Organon Logo, and HADLIMA are trademarks of N.V. Organon. All other trademarks appearing herein are trademarks of their respective owners.
Cautionary Note Regarding Forward-Looking Statements
Some statements and disclosures in this press release are “forward-looking statements” within the meaning of the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995. Forward-looking statements include all statements that do not relate solely to historical or current facts and can be identified by the use of words such as “may,” “expects,” “intends,” “anticipates,” “plans,” “believes,” “seeks,” “estimates,” “will,” or words of similar meaning. These forward-looking statements are based on Organon’s current plans and expectations and are subject to a number of risks and uncertainties that could cause Organon’s plans and expectations, including actual results, to differ materially from the forward-looking statements.
Risks and uncertainties that may affect Organon’s future results include, but are not limited to, an inability to fully execute on the product development and commercialization plans for HADLIMA in the United States due to factors outside of Organon’s reasonable control; efficacy, safety, or other quality concerns with respect to marketed products, including market actions such as recalls, withdrawals, or declining sales; political and social pressures, or regulatory developments, that adversely impact demand for, availability of, or patient access to Organon’s products; general economic factors, including recessionary pressures, interest rate and currency exchange rate fluctuations; general industry conditions and competition; the impact of the ongoing COVID-19 pandemic and emergence of variant strains; the impact of pharmaceutical industry regulation and health care legislation in the United States and internationally; global trends toward health care cost containment; technological advances; new products and patents attained by competitors; challenges inherent in new product development, including obtaining regulatory approval; Organon’s ability to accurately predict its future financial results and performance; manufacturing difficulties or delays; financial instability of international economies and sovereign risk; difficulties developing and sustaining relationships with commercial counterparties; dependence on the effectiveness of Organon’s patents and other protections for innovative products; and the exposure to litigation, including patent litigation, and/or regulatory actions.
Organon undertakes no obligation to publicly update any forward-looking statement, whether as a result of new information, future events or otherwise. Additional factors that could cause results to differ materially from those described in the forward-looking statements can be found in Organon’s filings with the Securities and Exchange Commission (“SEC”), including Organon’s Annual Report on Form 10-K for the year ended December 31, 2022, available at the SEC’s Internet site (www.sec.gov).
1 HADLIMA Prefilled syringe and autoinjector Ease of Use Certification. Arthritis Foundation. July 1, 2023. Accessed June 20, 2023. https://www.arthritis.org/partnership/ease-of-use-certification
View source version on businesswire.com: https://www.businesswire.com/news/home/20230701798272/en/
Contacts
Media Contacts – Organon
Karissa Peer, karissa.peer@organon.com
Hannah Silver, hannah.silver@organon.com
Media Contacts – Samsung Bioepis
Anna Nayun Kim, nayun86.kim@samsung.com
Jane Chung, ejane.chung@samsung.com
Source: Organon
HADLIMA (adalimumab-bwwd) PushTouch Autoinjector Close-up (Photo: Business Wire)
HADLIMA (adalimumab-bwwd) Prefilled Syringe (Photo: Business Wire)
HADLIMA (adalimumab-bwwd) PushTouch Autoinjector (Photo: Business Wire)
View this news release and multimedia online at:
http://www.businesswire.com/news/home/20230701798272/en