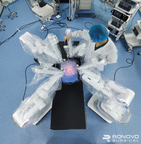
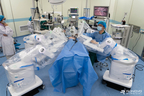
Ronovo Surgical invited top gynecology and urology surgeons to evaluate the performance of its newly unveiled Carina™ RAS Platform, the first modular robotic-assisted surgery platform developed in China for laparoscopic procedures.
SHANGHAI--(BUSINESS WIRE)-- Recently, Ronovo Surgical invited top gynecology and urology surgeons to evaluate the performance of its newly unveiled Carina™ RAS Platform, the first modular robotic-assisted surgery (RAS) platform developed in China for laparoscopic procedures.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20230301005036/en/

Image: Carina™ RAS Platform being configured for animal lab. (Photo: Business Wire)
The invited surgeons combine for more than 11,000 robotic surgeries that span both urology and gynecology. These experts included:
- Professor Dingwei Ye
- Vice Chairman, Fudan University Cancer Institute (Shanghai)
- Chairman, Urology Section, China Anti-Cancer Association
- Led and performed over 8000 robotic surgeries
- Professor Mei Ji
- Deputy Director, Department of Gynecology, First Affiliated Hospital of Zhengzhou University
- Performed over 2000 robotic surgeries
- Professor Dapeng Wu
- Deputy Director, Department of Urology, First Affiliated Hospital of Xi’an Jiaotong University
- Performed over 1000 robotic surgeries
Uniquely designed for optimized surgical access in broader surgical specialties, Carina™ features single-instrument robotic arms that are nimbly distributed around the operating bed based on the surgeon’s preferences. The small footprint of each bedside module is designed to improve the surgical team’s ability to efficiently utilize precious OR space. Additionally, individual modules can freely move to join others as needed in different operating rooms, enabling the platform to easily adapt to typical operating environments in China.
Carina™ allows laparoscopic surgeons to select the ideal types, quantities and configurations of surgical instruments based on their laparoscopy foundation. Such flexibility allows surgeons to leverage – rather than abandon – prior training and continue to improve upon their performance with minimal learning curve. This means broader utilization of Ronovo’s proprietary technology to enable MIS in more surgical specialties to be performed robotically.
While R&D efforts for Carina™ officially kicked off in 2021, Ronovo has developed multiple iterations in under two years and performed dozens of animal and cadaver labs, leading up to the recent animal lab series with gynecology and urology RAS experts.
The first evaluation in the live porcine series was performed by Prof. Ji from Zhengzhou University. With port placements set up like laparoscopy, she completed hysterectomy with bilateral salpingo-oophorectomy and iliac lymph node dissection. Numerous surgical maneuvers were precisely executed, including target exposure, dissection and coagulation of the uterine artery, transection of distal side of the cervix, and suturing of vaginal stump. The consecutive operations were performed uninterrupted and nearly free of bleeding. Prof. Ji also simulated pelvic lymphadenectomy to test ability to delicately dissect, coagulate and resect around vessels for complete exposure of internal and external iliac arteries. Commenting on her experience, Prof. Ji asserted that, “the completely modular design demonstrated exceptional operating capabilities during the live animal trial. I consider the Carina™ robotic platform and its instruments fully ready for human clinical trials.”
The second evaluation in the live series was led by Prof. Ye from Fudan University. On Carina’s modular architecture, he commented that the design can bring dexterity and practicality uniquely advantageous in a variety of MIS applications. On Carina’s imaging capability, he complimented on the clarity and vividness of the 3DHD stereoscopic view. His evaluation started with unilateral partial nephrectomy, where he successfully exposed and mobilized the renal artery, followed by renal tissue resection and suturing, and ended with simulated vesicourethral anastomosis. Commenting on his experience, Prof. Ye observed that, “by successfully completing several procedures with Carina™ and personally experiencing its performance, I am quite optimistic about its potential to excel in many clinical applications. Therefore, I fully support advancing Carina™ into human clinical trials as soon as possible.”
The final evaluation in the series was performed by Prof. Wu from Xi’an Jiaotong University. He was able to quickly familiarize with Carina™ and start performing complex and delicate surgical maneuvers. Four procedures were completed consecutively within three hours – partial nephrectomy, ureteroplasty, renal venotomy and suturing, as well as unilateral nephrectomy. Notably, renal warm ischemia time lasted only 10 minutes during partial nephrectomy. Commenting on his experience, Prof. Wu concluded that, “Carina™ provided surprisingly realistic stereoscopic vision, reliable hand control-to-instrument tracking, robust wristed instruments, and ergonomic comfort – all of which are important requirements for my surgeries.” Additionally, he pointed out the unique modular design’s potential to overcome challenges of performing multi-quadrant surgeries on existing surgical robots. Prof. Wu hopes to see Carina™ in routine clinical use as soon as possible to benefit MIS patients.
For almost two decades, the laparoscopic surgical robotics market in China has been a foreign monopoly. Although the clinical value of RAS is now well accepted in urologic minimally invasive surgeries (MIS), the prohibitive cost and sluggish expansion into additional specialties present substantial barriers to broader adoption. This major market gap inspired Ronovo to develop Carina™ to address practical needs of nearly 10 million MIS patients, approximately 120,000 laparoscopic surgeons, and more than 10,000 hospitals in China every year.
KOL feedback from these latest evaluations represents a major milestone in readiness for human clinical trials, which are planned for later this year. The company expects to commercialize Carina™ in 2024, starting with China and aiming to expand globally soon thereafter.
About Ronovo Surgical
Founded in 2019, Ronovo Surgical is accelerating the transition to truly intelligent MIS through innovative robotic and digital solutions. It is the only soft tissue robotic surgery company in China led by seasoned industry leaders with expertise in both commercial leadership and technology development. Founder, Chairman and CEO, John Ma, Ph.D., is former SVP at Intuitive and Global Partner at Fosun Group. Co-Founder, COO and CTO, Ying Mao, Ph.D., is former systems engineering lead at Auris Health (now J&J Ethicon) and founder of Dreamworld AR. With more than two decades of leadership experience combined specifically in the development and commercialization of surgical robotics systems, they now lead a seasoned core team that represents deep product development and implementation expertise in surgical robotics, industrial robotics, medical capital equipment, and medical consumables from Chinese and multinational manufacturers. Additionally, Ronovo has significant global clinical and technical resources, having established long-term partnerships with numerous clinical and technical advisors.
For more information, please visit: http://www.ronovosurgical.com/.
View source version on businesswire.com: https://www.businesswire.com/news/home/20230301005036/en/
Contacts
ZiHan Lin
VP of Business Development
zihan.lin@ronovosurgical.com
Source: Ronovo Surgical
Image: Carina™ RAS Platform being configured for animal lab. (Photo: Business Wire)
Image: Carina™ RAS Platform in action during animal lab series. (Photo: Business Wire)
View this news release and multimedia online at:
http://www.businesswire.com/news/home/20230301005036/en