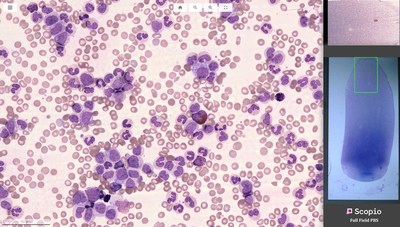
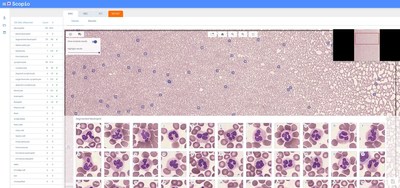
Scopio Labs, a leading provider of Full Field Morphology (FFM), announced today that it was granted FDA clearance to market and sell its X100 with Full Field Peripheral Blood Smear (Full Field PBS) Application, unlocking the potential of in vitro hematology diagnosis
|
TEL AVIV, Israel, Oct. 21, 2020 /PRNewswire/ -- Scopio Labs, a leading provider of Full Field Morphology (FFM), announced today that it was granted FDA clearance to market and sell its X100 with Full Field Peripheral Blood Smear (Full Field PBS) Application, unlocking the potential of in vitro hematology diagnosis. Full Field PBS is also available in Europe with CE mark certification granted earlier this year. Using advanced computational photography imaging and tailored AI tools, Full Field PBS gives clinical laboratories an unprecedented ability to capture digital scans with full field view of the monolayer and feathered edge at 100X oil immersion resolution level. The Full Field PBS utilizes adaptive monolayer identification in support of long and short smears and automates the analysis process by pre-classifying 200 white blood cells (WBC), providing platelet pre-estimate, and enabling RBC morphology evaluation. Blood is one of the most foundational gateways to health information. Roughly 120,000 laboratories worldwide conduct 600 million PBS tests annually for the global population, predominantly via manual microscopes. Even with the adoption of digital tools, today's solutions do not showcase all required regions of interest in a PBS slide, only capturing snapshots of cells. Consequently, technologists frequently default to the manual microscope for a more detailed examination of the raw data. "Understanding the challenges lab technicians, hematologists and hematopathologists face when evaluating blood samples containing large numbers of morphologically-unique cells in a timely fashion, we designed our solution specifically for hematology labs where we can improve quality of care, consistency of results and reduce review time," said Scopio Labs' CEO and Co-Founder, Itai Hayut. "We are thrilled to receive FDA clearance following the successful completion of a multi-center study, as we bring our innovative solution to laboratories around the U.S. to help improve the outcome of diagnosis and care." Full Field PBS is an end-to-end 100X oil immersion resolution digital microscopy solution for PBS which includes a three-slide tray and a decision support system (DSS) for pre-classification of WBC into 16 classes, red blood cell (RBC) morphology evaluations, platelet location and pre-estimate. It features adaptive monolayer identification for optimal imaging and analysis of short and long smears, including the feathered edge of the sample. With its fully digital, automated scan and image acquisition system, the Full Field PBS offers unique user experience of in-slide navigation to specified locations within a slide, all through a modern web browser interface. Click here to experience the Scopio Full Field PBS scan. "The field of microscopy is poised for transformation, and I am enthusiastic about the prospects of Scopio Labs' innovative application," said Michael D. Feldman, MD, PhD, Vice Chairman Clinical Services, Pathology and Laboratory Medicine at University of Pennsylvania School of Medicine. "With new digital technologies combining imaging and artificial intelligence being introduced into the laboratory, we can provide clinicians with tools that strengthen their capabilities." "With the first clinical-level solution that digitizes large portions of PBS, Scopio Labs is taking hematology through the next technological revolution with exponential impact to the industry, leveraging its unique computational imaging technology and specifically designed computer vision tools," said Erez Na'aman, co-founder and CTO of Scopio Labs. "We are transforming hematology diagnostics, empowering experts and propelling the field forward." Earlier this year, the company closed a $16 million Series B funding round, bringing total funding to $30 million. Scopio Labs is setting its sights on transforming all hematology applications, including bone marrow aspirates (BMA) and body fluids. With a robust product development pipeline, and the ability to detect morphological events on a cellular and subcellular scale, Scopio Labs will open the door for morphology-based diagnostics, disease monitoring and treatment adjustment for various blood cancers. About Scopio Labs Scopio Labs, co-founded by Itai Hayut and Erez Na'aman in 2015, is dedicated to unlocking the potential of imaging and AI in hematology, redefining diagnostics and related drug development by championing Full Field Morphology (FFM) at scale. Uncovering actionable insights from vast amounts of data readily available in the average blood sample and bone marrow aspirate, Scopio's solutions will aim to showcase thousands of cells at scale in high resolution, to enable new hematology diagnostic methods. Its Full Field Peripheral Blood Smear (Full Field PBS) application captures and digitizes microscopy data in high resolution and large scan areas, using advanced computational photography tools, and offers a cutting-edge decision support system (DSS). Full Field PBS is available for hematology labs across Europe and the United States with both CE mark certification and FDA clearance granted in 2020. The company's end-to-end solutions provide AI-based decision support systems and remote collaboration tools in hematology, research and veterinary medicine. It currently offers its advanced ScopioVet Digital Cytology System to veterinary clinics throughout the United States, United Kingdom and Ireland. Scopio supports clinical applications while also powering innovation in areas such as academic research and drug discovery. Scopio Labs has raised $30 million to date. Investors include Olive Tree Ventures, Aurum Ventures, OurCrowd, LR Group and others. The company operates out of its two offices in New Jersey and Tel Aviv. For more information visit our website, http://scopiolabs.com and follow the company on LinkedIn and Twitter. Media Contact:
SOURCE Scopio Labs |